Correlated evolution between targets of pre- and postcopulatory sexual selection across squamate reptiles
- PMID: 27777721
- PMCID: PMC5058519
- DOI: 10.1002/ece3.2344
Correlated evolution between targets of pre- and postcopulatory sexual selection across squamate reptiles
Abstract
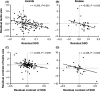
Sexual selection reflects the joint contributions of precopulatory selection, which arises from variance in mating success, and postcopulatory selection, which arises from variance in fertilization success. The relative importance of each episode of selection is variable among species, and comparative evidence suggests that traits targeted by precopulatory selection often covary in expression with those targeted by postcopulatory selection when assessed across species, although the strength and direction of this association varies considerably among taxa. We tested for correlated evolution between targets of pre- and postcopulatory selection using data on sexual size dimorphism (SSD) and testis size from 151 species of squamate reptiles (120 lizards, 31 snakes). In squamates, male-male competition for mating opportunities often favors large body size, such that the degree of male-biased SSD is associated with the intensity of precopulatory selection. Likewise, competition for fertilization often favors increased sperm production, such that testis size (relative to body size) is associated with the intensity of postcopulatory selection. Using both conventional and phylogenetically based analyses, we show that testis size consistently decreases as the degree of male-biased SSD increases across lizards and snakes. This evolutionary pattern suggests that strong precopulatory selection may often constrain the opportunity for postcopulatory selection and that the relative importance of each selective episode may determine the optimal resolution of energy allocation trade-offs between traits subject to each form of sexual selection.
Keywords: Phylogenetic comparative method; sexual size dimorphism; sperm competition; testis size.
Figures
Similar articles
-
Sperm competition and the evolution of precopulatory weapons: Testis size and amplexus position, but not arm strength, affect fertilization success in a chorusing frog.Evolution. 2017 Feb;71(2):329-341. doi: 10.1111/evo.13136. Epub 2016 Dec 23. Evolution. 2017. PMID: 27911018
-
Disentangling precopulatory and postcopulatory sexual selection in polyandrous species.Evolution. 2014 May;68(5):1320-31. doi: 10.1111/evo.12353. Epub 2014 Mar 4. Evolution. 2014. PMID: 24410424
-
Sexual size dimorphism and male reproductive traits vary across populations of a tropical rainforest dung beetle species (Onthophagus babirussa).Ecol Evol. 2022 Sep 16;12(9):e9279. doi: 10.1002/ece3.9279. eCollection 2022 Sep. Ecol Evol. 2022. PMID: 36177114 Free PMC article.
-
Sexual selection and sperm diversity in primates.Mol Cell Endocrinol. 2020 Dec 1;518:110974. doi: 10.1016/j.mce.2020.110974. Epub 2020 Sep 12. Mol Cell Endocrinol. 2020. PMID: 32926966 Review.
-
The evolution of male mate choice in insects: a synthesis of ideas and evidence.Biol Rev Camb Philos Soc. 2001 Aug;76(3):305-39. doi: 10.1017/s1464793101005693. Biol Rev Camb Philos Soc. 2001. PMID: 11569787 Review.
Cited by
-
Experimental manipulation of reproductive tactics in Seba's short-tailed bats: consequences on sperm quality and oxidative status.Curr Zool. 2019 Dec;65(6):609-616. doi: 10.1093/cz/zoz011. Epub 2019 Mar 23. Curr Zool. 2019. PMID: 31857807 Free PMC article.
-
Sexual ornaments but not weapons trade off against testes size in primates.Proc Biol Sci. 2019 Apr 10;286(1900):20182542. doi: 10.1098/rspb.2018.2542. Proc Biol Sci. 2019. PMID: 30966988 Free PMC article.
-
Sexual selection and sexual size dimorphism in animals.Biol Lett. 2021 Sep;17(9):20210251. doi: 10.1098/rsbl.2021.0251. Epub 2021 Sep 15. Biol Lett. 2021. PMID: 34520680 Free PMC article.
-
Measuring Pre- and Post-Copulatory Sexual Selection and Their Interaction in Socially Monogamous Species with Extra-Pair Paternity.Cells. 2021 Mar 11;10(3):620. doi: 10.3390/cells10030620. Cells. 2021. PMID: 33799610 Free PMC article.
-
Immune stress and diet influence reproductive fitness in male tuatara (Sphenodon punctatus).Curr Zool. 2024 Apr 3;70(6):786-794. doi: 10.1093/cz/zoae012. eCollection 2024 Dec. Curr Zool. 2024. PMID: 39678825 Free PMC article.
References
-
- Blomberg, S. P. , Garland T., and Ives A. R.. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. - PubMed
-
- Calsbeek, R. , Bonneaud C., Prabhu S., Manoukis N., and Smith T. B.. 2007. Multiple paternity and sperm storage lead to increased genetic diversity in Anolis lizards. Evol. Ecol. 9:495–503.
-
- Carothers, J. H. 1984. Sexual selection and sexual dimorphism in some herbivorous lizards. Am. Nat. 124:244–254.
-
- Cox, R. M. , and Kahrl A. F.. 2014. Sexual selection and sexual dimorphism Pp. 78–108 in Rheubert J. L., Siegel D. S., Trauth S. E. and Jamieson B. G., eds. Reproductive biology and phylogeny of lizards and tuatara. CRC Press, Boca Raton, FL.
LinkOut - more resources
Full Text Sources
Other Literature Sources

