Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors
- PMID: 27777771
- PMCID: PMC5067894
- DOI: 10.1186/s40425-016-0164-7
Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors
Abstract
Background: We evaluated whether tumor infiltrating lymphocytes (TIL) could be expanded from surgically resected tumors from pancreatic cancer patients.
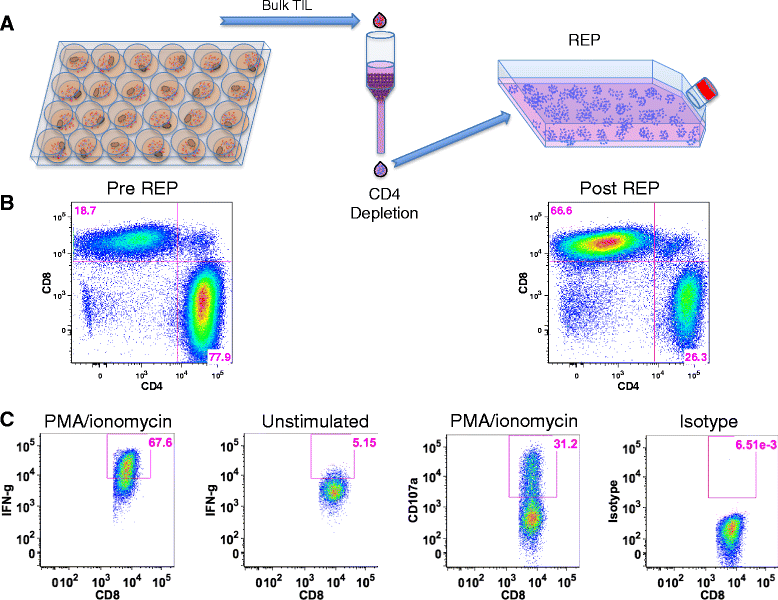
Methods: Tumors were resected from pancreatic cancer patients. Tumors were minced into fragments and cultured in media containing high dose interleukin-2 (IL-2) for up to 6 weeks. T cell phenotype, activation markers, and reactivity were measured.
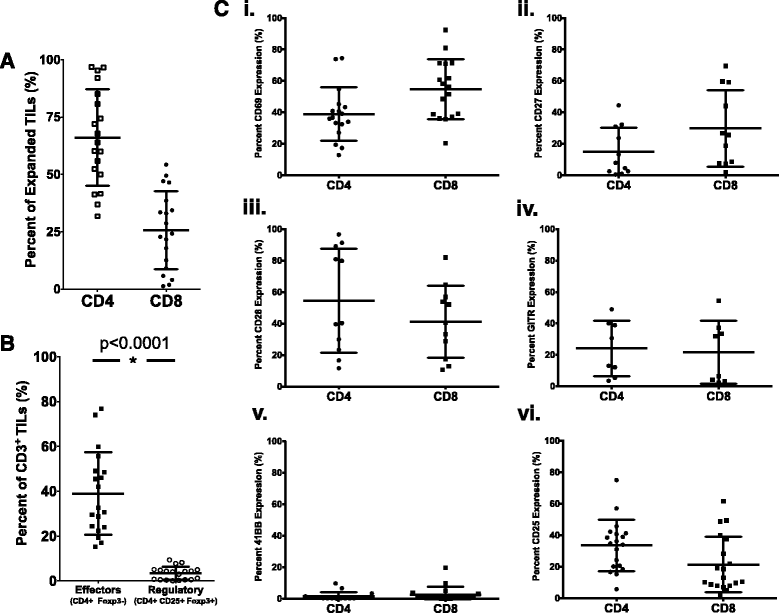
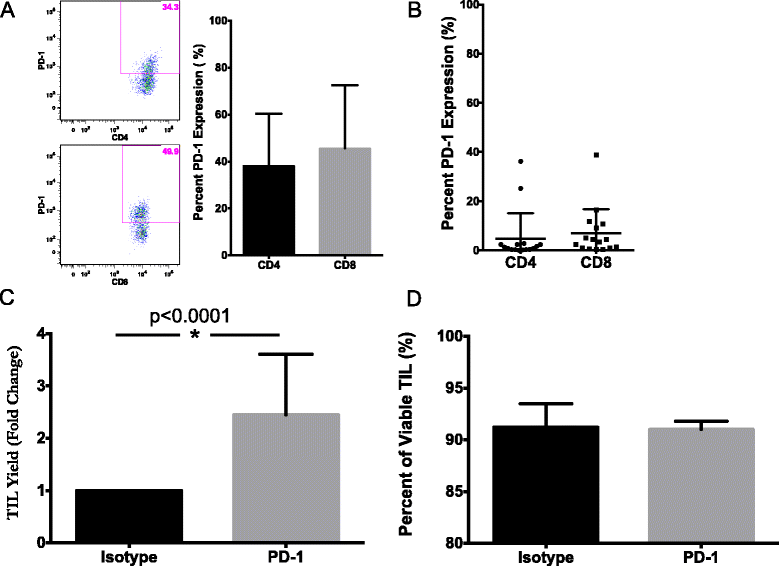
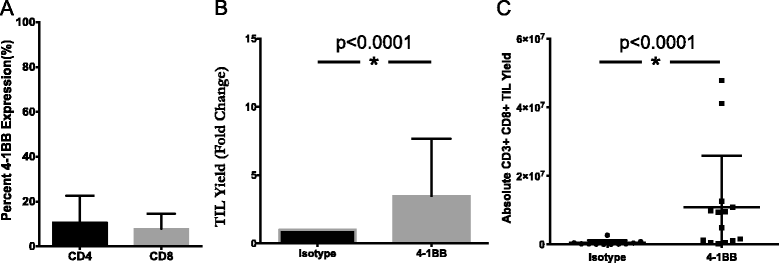
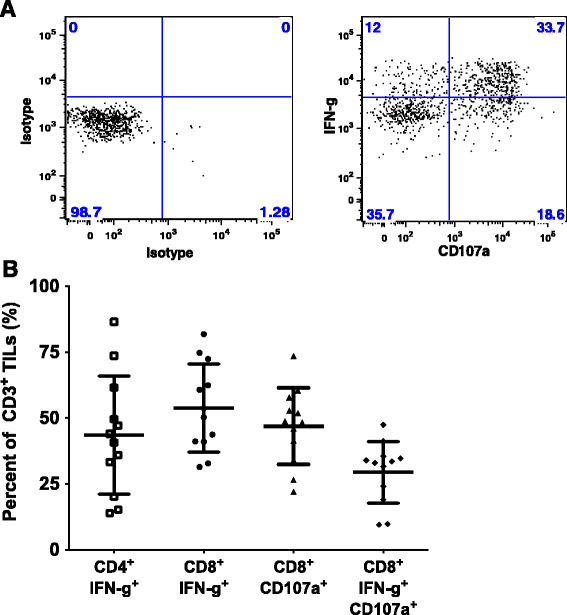
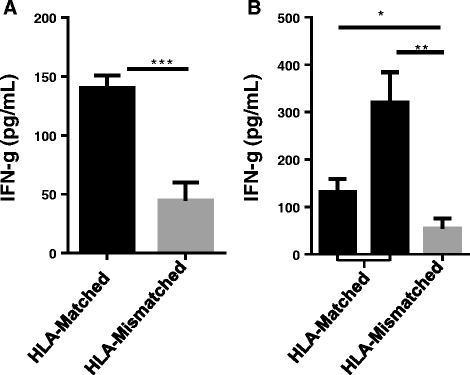
Results: TIL expansion was measured in 19 patient samples. The majority of these TIL were CD4+ T cells and were highly activated. Purified CD8+ T cells produced IFN-γ in response to HLA-matched pancreatic tumor targets. PD-1 blockade and 4-1BB stimulation were demonstrated as effective strategies to improve effective TIL yield, including the production of tumor-reactive pancreatic TIL.
Conclusions: TIL expanded from pancreatic tumors are functional and able to respond to pancreatic tumor associated antigens. PD-1 blockade, 41BB stimulation, and CD8+ T cell enrichment are effective strategies to improve TIL yield and tumor reactivity. These results support the development of adoptive cell therapy strategies using TIL for the treatment of pancreatic cancer.
Keywords: Adoptive cell therapy; Pancreatic cancer; T cells; Tumor infiltrating lymphocytes (TIL).
Figures
References
-
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfew immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. - DOI - PMC - PubMed
-
- Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F, Isambert N, Chaigneau L, Le Brun-Ly V, Dubreuil P, Cremer I, Caignard A, Poirier-Colame V, Chaba K, Flament C, Halama N, Jäger D, Eggermont A, Bonvalot S, Commo F, Terrier P, Opolon P, Emile JF, Coindre JM, Kroemer G, Chaput N, Le Cesne A, Blay JY, Zitvogel L. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73(12):3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
