Acute visual loss after ipilimumab treatment for metastatic melanoma
- PMID: 27777775
- PMCID: PMC5067900
- DOI: 10.1186/s40425-016-0170-9
Acute visual loss after ipilimumab treatment for metastatic melanoma
Abstract
Background: Ipilimumab, a humanized CLTA-4 antibody is a standard therapy in the treatment of advanced melanoma. While ipilimumab provides an overall survival benefit to patients, it can be associated with immune related adverse events (IrAEs).
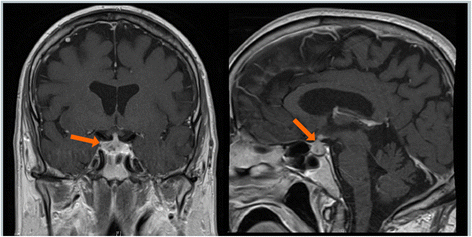
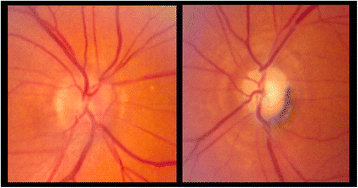
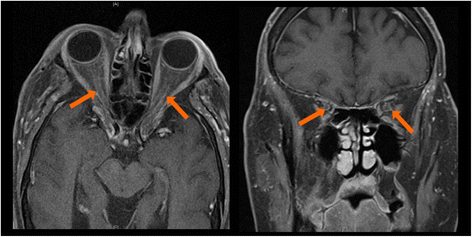
Case presentation: Here we describe a patient treated with ipilimumab who experienced known IrAEs, including hypophysitis, as well as a profound vision loss due to optic neuritis. There are rare reports of optic neuritis occurring as an adverse event associated with ipilimumab treatment. Furthermore, the patient experienced multiple complications from high dose steroids used to manage his IrAEs.
Conclusions: This case highlights the need for recognition of atypical immune mediated processes associated with newer checkpoint inhibitor therapies including ipilimumab.
Keywords: Checkpoint inhibitors; Immune; Ipilimumab; Melanoma; Optic neuritis; Side effects.
Figures
Similar articles
-
Ipilimumab may increase the severity of cutenaous toxicity related to radiotherapy.J Oncol Pharm Pract. 2016 Jun;22(3):533-6. doi: 10.1177/1078155215572930. Epub 2015 Feb 17. J Oncol Pharm Pract. 2016. PMID: 25694346
-
Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy.J Immunother Cancer. 2017 Feb 21;5:8. doi: 10.1186/s40425-017-0210-0. eCollection 2017. J Immunother Cancer. 2017. PMID: 28239462 Free PMC article.
-
Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis.Oncologist. 2017 Jun;22(6):709-718. doi: 10.1634/theoncologist.2016-0487. Epub 2017 May 11. Oncologist. 2017. PMID: 28495807 Free PMC article.
-
Challenging Cases: Management of Immune-Related Toxicity.Am Soc Clin Oncol Educ Book. 2018 May 23;38:179-183. doi: 10.1200/EDBK_209557. Am Soc Clin Oncol Educ Book. 2018. PMID: 30231403 Review.
-
Ipilimumab-induced necrotic myelopathy in a patient with metastatic melanoma: A case report and review of literature.J Oncol Pharm Pract. 2016 Jun;22(3):537-42. doi: 10.1177/1078155215572932. Epub 2015 Feb 23. J Oncol Pharm Pract. 2016. PMID: 25712627 Review.
Cited by
-
Factors associated with ocular adverse event after immune checkpoint inhibitor treatment.Cancer Immunol Immunother. 2020 Dec;69(12):2441-2452. doi: 10.1007/s00262-020-02635-3. Epub 2020 Jun 17. Cancer Immunol Immunother. 2020. PMID: 32556494 Free PMC article.
-
The neurotoxic effects of immune checkpoint inhibitor therapy for melanoma.Melanoma Manag. 2019 May 31;6(2):MMT16. doi: 10.2217/mmt-2019-0001. Melanoma Manag. 2019. PMID: 31406561 Free PMC article. Review. No abstract available.
-
Neurological Complications of Conventional and Novel Anticancer Treatments.Cancers (Basel). 2022 Dec 10;14(24):6088. doi: 10.3390/cancers14246088. Cancers (Basel). 2022. PMID: 36551575 Free PMC article. Review.
-
Pembrolizumab-induced optic neuropathy - a case report.Front Immunol. 2023 May 9;14:1171981. doi: 10.3389/fimmu.2023.1171981. eCollection 2023. Front Immunol. 2023. PMID: 37228591 Free PMC article.
-
Neurologic Immune-Related Adverse Events Associated with Immune Checkpoint Inhibition.Curr Oncol Rep. 2019 Nov 27;21(12):108. doi: 10.1007/s11912-019-0859-2. Curr Oncol Rep. 2019. PMID: 31776691 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
