FoxO1 interacts with transcription factor EB and differentially regulates mitochondrial uncoupling proteins via autophagy in adipocytes
- PMID: 27777789
- PMCID: PMC5046220
- DOI: 10.1038/cddiscovery.2016.66
FoxO1 interacts with transcription factor EB and differentially regulates mitochondrial uncoupling proteins via autophagy in adipocytes
Abstract
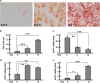
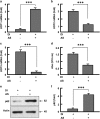
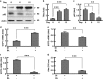
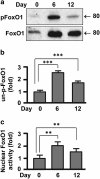
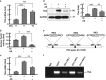
Mitochondrial uncoupling proteins (UCPs) are inducible and play an important role in metabolic and redox homeostasis. Recent studies have suggested that FoxO1 controls mitochondrial biogenesis and morphology, but it remains largely unknown how FoxO1 may regulate mitochondrial UCPs. Here we show that FoxO1 interacted with transcription factor EB (Tfeb), a key regulator of autophagosome and lysosome, and mediated the expression of UCP1, UCP2 and UCP3 differentially via autophagy in adipocytes. UCP1 was down-regulated but UCP2 and UCP3 were upregulated during adipocyte differentiation, which was associated with increased Tfeb and autophagy activity. However, inhibition of FoxO1 suppressed Tfeb and autophagy, attenuating UCP2 and UCP3 but increasing UCP1 expression. Pharmacological blockade of autophagy recapitulated the effects of FoxO1 inhibition on UCPs. Chromatin immunoprecipitation assay demonstrated that FoxO1 interacted with Tfeb by directly binding to its promoter, and silencing FoxO1 led to drastic decrease in Tfeb transcript and protein levels. These data provide the first line of evidence that FoxO1 interacts with Tfeb to regulate autophagy and UCP expression in adipocytes. Dysregulation of FoxO1→autophagy→UCP pathway may account for metabolic changes in obesity.
Figures
Similar articles
-
Genomic organization and mutational analysis of the human UCP2 gene, a prime candidate gene for human obesity.J Recept Signal Transduct Res. 1999 Jan-Jul;19(1-4):229-44. doi: 10.3109/10799899909036648. J Recept Signal Transduct Res. 1999. PMID: 10071761
-
Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy.Autophagy. 2019 Jan;15(1):131-150. doi: 10.1080/15548627.2018.1511263. Epub 2018 Sep 13. Autophagy. 2019. PMID: 30209975 Free PMC article.
-
UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency.Biochim Biophys Acta. 2001 Mar 1;1504(1):82-106. doi: 10.1016/s0005-2728(00)00247-4. Biochim Biophys Acta. 2001. PMID: 11239487 Review.
-
Acute and Chronic Ethanol Administration Differentially Modulate Hepatic Autophagy and Transcription Factor EB.Alcohol Clin Exp Res. 2015 Dec;39(12):2354-63. doi: 10.1111/acer.12904. Epub 2015 Nov 11. Alcohol Clin Exp Res. 2015. PMID: 26556759
-
UCP1: the original uncoupling protein--and perhaps the only one? New perspectives on UCP1, UCP2, and UCP3 in the light of the bioenergetics of the UCP1-ablated mice.J Bioenerg Biomembr. 1999 Oct;31(5):475-91. doi: 10.1023/a:1005400507802. J Bioenerg Biomembr. 1999. PMID: 10653476 Review.
Cited by
-
The adrenergic-induced ERK3 pathway drives lipolysis and suppresses energy dissipation.Genes Dev. 2020 Apr 1;34(7-8):495-510. doi: 10.1101/gad.333617.119. Epub 2020 Mar 5. Genes Dev. 2020. PMID: 32139423 Free PMC article.
-
TFEB: A Emerging Regulator in Lipid Homeostasis for Atherosclerosis.Front Physiol. 2021 Feb 17;12:639920. doi: 10.3389/fphys.2021.639920. eCollection 2021. Front Physiol. 2021. PMID: 33679452 Free PMC article. Review.
-
The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients.Cancers (Basel). 2023 Apr 24;15(9):2436. doi: 10.3390/cancers15092436. Cancers (Basel). 2023. PMID: 37173902 Free PMC article.
-
Fibroblast growth factor 21 (FGF21) attenuates tacrolimus-induced hepatic lipid accumulation through transcription factor EB (TFEB)-regulated lipophagy.J Zhejiang Univ Sci B. 2023 Jun 15;24(6):485-495. doi: 10.1631/jzus.B2200562. J Zhejiang Univ Sci B. 2023. PMID: 37309040 Free PMC article.
-
Diabetes and dyslipidemia: characterizing lipoprotein metabolism.Diabetes Metab Syndr Obes. 2017 Jul 28;10:333-343. doi: 10.2147/DMSO.S115855. eCollection 2017. Diabetes Metab Syndr Obes. 2017. PMID: 28814891 Free PMC article. Review.
References
-
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203–209. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

