Crucial Contributions by T Lymphocytes (Effector, Regulatory, and Checkpoint Inhibitor) and Cytokines (TH1, TH2, and TH17) to a Pathological Complete Response Induced by Neoadjuvant Chemotherapy in Women with Breast Cancer
- PMID: 27777963
- PMCID: PMC5061970
- DOI: 10.1155/2016/4757405
Crucial Contributions by T Lymphocytes (Effector, Regulatory, and Checkpoint Inhibitor) and Cytokines (TH1, TH2, and TH17) to a Pathological Complete Response Induced by Neoadjuvant Chemotherapy in Women with Breast Cancer
Abstract
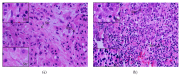
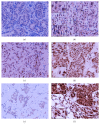
The tumour microenvironment consists of malignant cells, stroma, and immune cells. Prominent tumour-infiltrating lymphocytes (TILs) in breast cancer are associated with a good prognosis and are predictors of a pathological complete response (pCR) with neoadjuvant chemotherapy (NAC). The contribution of different T effector/regulatory cells and cytokines to tumour cell death with NAC requires further characterisation and was investigated in this study. Breast tumours from 33 women with large and locally advanced breast cancers undergoing NAC were immunohistochemically (intratumoural, stromal) assessed for T cell subsets and cytokine expression using labelled antibodies, employing established semiquantitative methods. Prominent levels of TILs and CD4+, CD8+, and CTLA-4+ (stromal) T cells and CD8+ : FOXP3+ ratios were associated with a significant pCR; no association was seen with FOXP3+, CTLA-4+ (intratumoural), and PD-1+ T cells. NAC significantly reduced CD4+, FOXP3+, CTLA-4+ (stromal) (concurrently blood FOXP3+, CTLA-4+ Tregs), and PD-1+ T cells; no reduction was seen with CD8+ and CTLA-4+ (intratumoural) T cells. High post-NAC tumour levels of FOXP3+ T cells, IL-10, and IL-17 were associated with a failed pCR. Our study has characterised further the contribution of T effector/regulatory cells and cytokines to tumour cell death with NAC.
Figures





References
-
- Aloysius M., Walker L., Eremin O. Cancer and the immune response. In: Eremin O., Sewell H., editors. Essential Immunology for Surgeons. chapter 4. Oxford, UK: OUP; 2011. pp. 237–302.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

