Improving vascular maturation using noncoding RNAs increases antitumor effect of chemotherapy
- PMID: 27777972
- PMCID: PMC5070952
- DOI: 10.1172/jci.insight.87754
Improving vascular maturation using noncoding RNAs increases antitumor effect of chemotherapy
Erratum in
-
Improving vascular maturation using noncoding RNAs increases antitumor effect of chemotherapy.JCI Insight. 2021 Apr 8;6(7):e149896. doi: 10.1172/jci.insight.149896. JCI Insight. 2021. PMID: 33793423 Free PMC article. No abstract available.
Expression of concern in
-
Improving vascular maturation using noncoding RNAs increases antitumor effect of chemotherapy.JCI Insight. 2018 Jun 7;3(11):e122387. doi: 10.1172/jci.insight.122387. eCollection 2018 Jun 7. JCI Insight. 2018. PMID: 29889661 Free PMC article. No abstract available.
Abstract
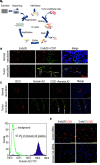
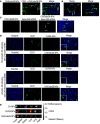
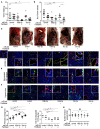
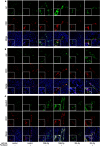
Current antiangiogenesis therapy relies on inhibiting newly developed immature tumor blood vessels and starving tumor cells. This strategy has shown transient and modest efficacy. Here, we report a better approach to target cancer-associated endothelial cells (ECs), reverse permeability and leakiness of tumor blood vessels, and improve delivery of chemotherapeutic agents to the tumor. First, we identified deregulated microRNAs (miRs) from patient-derived cancer-associated ECs. Silencing these miRs led to decreased vascular permeability and increased maturation of blood vessels. Next, we screened a thioaptamer (TA) library to identify TAs selective for tumor-associated ECs. An annexin A2-targeted TA was identified and used for delivery of miR106b-5p and miR30c-5p inhibitors, resulting in vascular maturation and antitumor effects without inducing hypoxia. These findings could have implications for improving vascular-targeted therapy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

