Lymphocyte activation gene 3 and coronary artery disease
- PMID: 27777974
- PMCID: PMC5070966
- DOI: 10.1172/jci.insight.88628
Lymphocyte activation gene 3 and coronary artery disease
Abstract
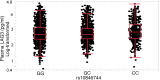
BACKGROUND: The lipoprotein scavenger receptor BI (SCARB1) rs10846744 noncoding variant is significantly associated with atherosclerotic disease independently of traditional cardiovascular risk factors. We identified a potentially novel connection between rs10846744, the immune checkpoint inhibitor lymphocyte activation gene 3 (LAG3), and atherosclerosis. METHODS: In vitro approaches included flow cytometry, lipid raft isolation, phosphosignaling, cytokine measurements, and overexpressing and silencing LAG3 protein. Fasting plasma LAG3 protein was measured in hyperalphalipoproteinemic (HALP) and Multi-Ethnic Study of Atherosclerosis (MESA) participants. RESULTS: In comparison with rs10846744 reference (GG homozygous) cells, LAG3 protein levels by flow cytometry (P < 0.001), in lipid rafts stimulated and unstimulated (P = 0.03), and phosphosignaling downstream of B cell receptor engagement of CD79A (P = 0.04), CD19 (P = 0.04), and LYN (P = 0.001) were lower in rs10846744 risk (CC homozygous) cells. Overexpressing LAG3 protein in risk cells and silencing LAG3 in reference cells confirmed its importance in phosphosignaling. Secretion of TNF-α was higher (P = 0.04) and IL-10 was lower (P = 0.04) in risk cells. Plasma LAG3 levels were lower in HALP carriers of the CC allele (P < 0.0001) and by race (P = 0.004). In MESA, race (P = 0.0005), age (P = 0.003), lipid medications (P = 0.03), smoking history (P < 0.0001), and rs10846744 genotype (P = 0.002) were independent predictors of plasma LAG3. In multivariable regression models, plasma LAG3 was significantly associated with HDL-cholesterol (HDL-C) (P = 0.007), plasma IL-10 (P < 0.0001), and provided additional predictive value above the Framingham risk score (P = 0.04). In MESA, when stratified by high HDL-C, plasma LAG3 was associated with coronary heart disease (CHD) (odds ratio 1.45, P = 0.004). CONCLUSION: Plasma LAG3 is a potentially novel independent predictor of HDL-C levels and CHD risk. FUNDING: This work was supported by an NIH RO1 grant (HL075646), the endowed Linda and David Roth Chair for Cardiovascular Research, and the Harold S. Geneen Charitable Trust Coronary Heart Disease Research award to Annabelle Rodriguez. MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-001079, UL1-TR-000040, and DK063491. Cardiometabochip genotyping data for the MESA samples was supported in part by grants and contracts R01HL98077, N02-HL-64278, HL071205, UL1TR000124, DK063491, RD831697, and P50 ES015915.
Figures







References
-
- Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94(23):12610–12615. doi: 10.1073/pnas.94.23.12610. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

