The role for neutrophil extracellular traps in cystic fibrosis autoimmunity
- PMID: 27777975
- PMCID: PMC5070963
- DOI: 10.1172/jci.insight.88912
The role for neutrophil extracellular traps in cystic fibrosis autoimmunity
Abstract
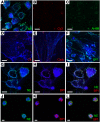
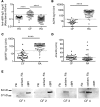
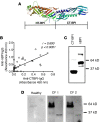
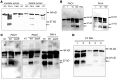
While respiratory failure in cystic fibrosis (CF) frequently associates with chronic infection by Pseudomonas aeruginosa, no single factor predicts the extent of lung damage in CF. To elucidate other causes, we studied the autoantibody profile in CF and rheumatoid arthritis (RA) patients, given the similar association of airway inflammation and autoimmunity in RA. Even though we observed that bactericidal permeability-increasing protein (BPI), carbamylated proteins, and citrullinated proteins all localized to the neutrophil extracellular traps (NETs), which are implicated in the development of autoimmunity, our study demonstrates striking autoantibody specificity in CF. Particularly, CF patients developed anti-BPI autoantibodies but hardly any anti-citrullinated protein autoantibodies (ACPA). In contrast, ACPA-positive RA patients exhibited no reactivity with BPI. Interestingly, anti-carbamylated protein autoantibodies (ACarPA) were found in both cohorts but did not cross-react with BPI. Contrary to ACPA and ACarPA, anti-BPI autoantibodies recognized the BPI C-terminus in the absence of posttranslational modifications. In fact, we discovered that P. aeruginosa-mediated NET formation results in BPI cleavage by P. aeruginosa elastase, which suggests a novel mechanism in the development of autoimmunity to BPI. In accordance with this model, autoantibodies associated with presence of P. aeruginosa on sputum culture. Finally, our results provide a role for autoimmunity in CF disease severity, as autoantibody levels associate with diminished lung function.
Figures
Similar articles
-
Dissociation of systemic and mucosal autoimmunity in cystic fibrosis.J Cyst Fibros. 2020 Mar;19(2):196-202. doi: 10.1016/j.jcf.2019.06.006. Epub 2019 Jun 28. J Cyst Fibros. 2020. PMID: 31262645 Free PMC article.
-
"NETtling" the host: Breaking of tolerance in chronic inflammation and chronic infection.J Autoimmun. 2018 Mar;88:1-10. doi: 10.1016/j.jaut.2017.10.008. J Autoimmun. 2018. PMID: 29100671 Review.
-
Anti-neutrophil cytoplasmic antibodies (ANCA) against bactericidal/permeability-increasing protein (BPI) and cystic fibrosis lung disease.Clin Exp Immunol. 1999 Sep;117(3):561-7. doi: 10.1046/j.1365-2249.1999.01006.x. Clin Exp Immunol. 1999. PMID: 10469063 Free PMC article.
-
Pseudomonas-induced lung damage in cystic fibrosis correlates to bactericidal-permeability increasing protein (BPI)-autoantibodies.Clin Exp Rheumatol. 2003 Nov-Dec;21(6 Suppl 32):S95-100. Clin Exp Rheumatol. 2003. PMID: 14740434
-
From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI).Autoimmun Rev. 2007 Mar;6(4):223-7. doi: 10.1016/j.autrev.2006.08.005. Epub 2006 Sep 5. Autoimmun Rev. 2007. PMID: 17317612 Review.
Cited by
-
Exploring key genes associated with neutrophil function and neutrophil extracellular traps in heart failure: a comprehensive analysis of single-cell and bulk sequencing data.Front Cell Dev Biol. 2023 Oct 24;11:1258959. doi: 10.3389/fcell.2023.1258959. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37941896 Free PMC article.
-
The Diethylcarbamazine Delays and Decreases the NETosis of Polymorphonuclear Cells of Humans with DM Type 2.J Diabetes Res. 2020 Mar 2;2020:4827641. doi: 10.1155/2020/4827641. eCollection 2020. J Diabetes Res. 2020. PMID: 32190698 Free PMC article.
-
Discovery of new candidate genes for rheumatoid arthritis through integration of genetic association data with expression pathway analysis.Arthritis Res Ther. 2017 Feb 2;19(1):19. doi: 10.1186/s13075-017-1220-5. Arthritis Res Ther. 2017. PMID: 28148290 Free PMC article.
-
Neutrophil Extracellular Traps as Prognostic Markers in COVID-19: A Welcome Piece to the Puzzle.Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):995-998. doi: 10.1161/ATVBAHA.120.315633. Epub 2021 Jan 27. Arterioscler Thromb Vasc Biol. 2021. PMID: 33955780 Free PMC article. No abstract available.
-
Targeting IgG Autoantibodies for Improved Cytotoxicity of Bactericidal Permeability Increasing Protein in Cystic Fibrosis.Front Pharmacol. 2020 Jul 17;11:1098. doi: 10.3389/fphar.2020.01098. eCollection 2020. Front Pharmacol. 2020. PMID: 32765284 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

