IP3 receptors regulate vascular smooth muscle contractility and hypertension
- PMID: 27777977
- PMCID: PMC5070959
- DOI: 10.1172/jci.insight.89402
IP3 receptors regulate vascular smooth muscle contractility and hypertension
Abstract
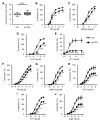
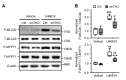
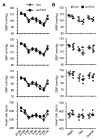
Inositol 1, 4, 5-trisphosphate receptor-mediated (IP3R-mediated) calcium (Ca2+) release has been proposed to play an important role in regulating vascular smooth muscle cell (VSMC) contraction for decades. However, whether and how IP3R regulates blood pressure in vivo remains unclear. To address these questions, we have generated a smooth muscle-specific IP3R triple-knockout (smTKO) mouse model using a tamoxifen-inducible system. In this study, the role of IP3R-mediated Ca2+ release in adult VSMCs on aortic vascular contractility and blood pressure was assessed following tamoxifen induction. We demonstrated that deletion of IP3Rs significantly reduced aortic contractile responses to vasoconstrictors, including phenylephrine, U46619, serotonin, and endothelin 1. Deletion of IP3Rs also dramatically reduced the phosphorylation of MLC20 and MYPT1 induced by U46619. Furthermore, although the basal blood pressure of smTKO mice remained similar to that of wild-type controls, the increase in systolic blood pressure upon chronic infusion of angiotensin II was significantly attenuated in smTKO mice. Taken together, our results demonstrate an important role for IP3R-mediated Ca2+ release in VSMCs in regulating vascular contractility and hypertension.
Figures


References
-
- Conlin PR, Williams GH. Use of calcium channel blockers in hypertension. Adv Intern Med. 1998;43:533–562. - PubMed
-
- Cummings DM, Amadio P, Nelson L, Fitzgerald JM. The role of calcium channel blockers in the treatment of essential hypertension. Arch Intern Med. 1991;151(2):250–259. - PubMed
-
- De Smedt H, et al. Determination of relative amounts of inositol trisphosphate receptor mRNA isoforms by ratio polymerase chain reaction. J Biol Chem. 1994;269(34):21691–21698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

