The head and neck cancer immune landscape and its immunotherapeutic implications
- PMID: 27777979
- PMCID: PMC5070962
- DOI: 10.1172/jci.insight.89829
The head and neck cancer immune landscape and its immunotherapeutic implications
Abstract
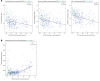
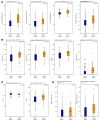
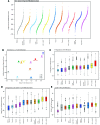
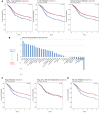
Recent clinical trials have demonstrated a clear survival advantage in advanced head and neck squamous cell carcinoma (HNSCC) patients treated with immune checkpoint blockade. These emerging results reveal that HNSCC is one of the most promising frontiers for immunotherapy research. However, further progress in head and neck immuno-oncology will require a detailed understanding of the immune infiltrative landscape found in these tumors. We leveraged transcriptome data from 280 tumors profiled by The Cancer Genome Atlas (TCGA) to comprehensively characterize the immune landscape of HNSCC in order to develop a rationale for immunotherapeutic strategies in HNSCC and guide clinical investigation. We find that both HPV+ and HPV- HNSCC tumors are among the most highly immune-infiltrated cancer types. Strikingly, HNSCC had the highest median Treg/CD8+ T cell ratio and the highest levels of CD56dim NK cell infiltration, in our pan-cancer analysis of the most immune-infiltrated tumors. CD8+ T cell infiltration and CD56dim NK cell infiltration each correlated with superior survival in HNSCC. Tumors harboring genetic smoking signatures had lower immune infiltration and were associated with poorer survival, suggesting these patients may benefit from immune agonist therapy. These findings illuminate the immune landscape of HPV+ and HPV- HNSCC. Additionally, this landscape provides a potentially novel rationale for investigation of agents targeting modulators of Tregs (e.g., CTLA-4, GITR, ICOS, IDO, and VEGFA) and NK cells (e.g., KIR, TIGIT, and 4-1BB) as adjuncts to anti-PD-1 in the treatment of advanced HNSCC.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

