Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression
- PMID: 27778363
- PMCID: PMC5216447
- DOI: 10.1111/apt.13833
Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression
Abstract
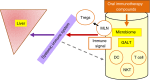
Background: The systemic immune system plays a role in inflammation and fibrogenesis associated with non-alcoholic steatohepatitis (NASH) and has become a potential target for drug development. In particular, the gut immune system has been suggested as a means for generating signals that can target the systemic immune system.
Aim: To describe seven novel methods being developed for the treatment of NASH that target the gut immune system for alleviation of the systemic inflammatory response, including oral administration of fatty-liver-derived proteins, anti-CD3 antibodies, tumour necrosis factor fusion protein, anti-lipopolysaccharide antibodies, glucosylceramide, delayed-release mercaptopurine, and soy-derived extracts.
Methods: A search for these methods for oral immunotherapy for NASH was conducted.
Results: Oral administration of these compounds provides an opportunity for immune modulation without immune suppression, with the advantage of being independent of a single molecular/inflammatory pathway. These modes of oral immune therapy demonstrate superior safety profiles, such that the patient is not exposed to general immune suppression. Moreover, these approaches target the whole spectrum of the disease and may serve as adjuvants to other therapies, such that they provide a platform for treatment of concomitant disorders in patients with NASH, including diabetes and hyperlipidaemia. Most of the compounds reviewed are currently in phase II trials, and it is anticipated that the acquisition of more clinical data in the next few years will enable the use of this new class of drugs for the treatment of NASH.
Conclusion: Oral immunotherapy may provide a novel platform for the treatment of NASH.
© 2016 The Author. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

References
-
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013; 10: 656–65. - PubMed
-
- Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis‐new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 2013; 10: 627–36. - PubMed
-
- Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol 2015; 62(1 Suppl): S65–75. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
