Effects of 12 Months of Caloric Restriction on Muscle Mitochondrial Function in Healthy Individuals
- PMID: 27778643
- PMCID: PMC5413108
- DOI: 10.1210/jc.2016-3211
Effects of 12 Months of Caloric Restriction on Muscle Mitochondrial Function in Healthy Individuals
Abstract
Context: The effects of caloric restriction (CR) on in vivo muscle mitochondrial function in humans are controversial.
Objective: We evaluated muscle mitochondrial function and associated transcriptional profiles in nonobese humans after 12 months of CR.
Design: Individuals from an ancillary study of the CALERIE 2 randomized controlled trial were assessed at baseline and 12 months after a 25% CR or ad libitum (control) diet.
Setting: The study was performed at Pennington Biomedical Research Center in Baton Rouge, LA.
Participants: Study participants included 51 (34 female subjects, 25 to 50 years of age) healthy nonobese individuals randomized to 1 of 2 groups (CR or control).
Intervention: This study included 12 months of a 25% CR or ad libitum (control) diet.
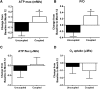
Main outcomes: In vivo mitochondrial function [maximal ATP synthesis rate (ATPmax), ATPflux/O2 (P/O)] was determined by 31P-magnetic resonance spectroscopy and optical spectroscopy, and body composition was determined by dual-energy X-ray absorptiometry. In a subset of individuals, a muscle biopsy was performed for transcriptional profiling via quantitative reverse transcription polymerase chain reaction and microarrays.
Results: Weight, body mass index (BMI), fat, and fat-free mass (P < 0.001 for all) significantly decreased at month 12 after CR vs control. In vivo ATPmax and P/O were unaffected by 12 months of CR. Targeted transcriptional profiling showed no effects on pathways involved in mitochondrial biogenesis, function, or oxidative stress. A subgroup analysis according to baseline P/O demonstrated that a higher (vs lower) P/O was associated with notable improvements in ATPmax and P/O after CR.
Conclusions: In healthy nonobese humans, CR has no effect on muscle mitochondrial function; however, having a "more coupled" (versus "less coupled") phenotype enables CR-induced improvements in muscle mitochondrial function.
Trial registration: ClinicalTrials.gov NCT00427193 NCT02695511.
Copyright © 2017 by the Endocrine Society
Figures

Similar articles
-
Differences in Mitochondrial Coupling Reveal a Novel Signature of Mitohormesis in Muscle of Healthy Individuals.J Clin Endocrinol Metab. 2016 Dec;101(12):4994-5003. doi: 10.1210/jc.2016-2742. Epub 2016 Oct 6. J Clin Endocrinol Metab. 2016. PMID: 27710240 Free PMC article. Clinical Trial.
-
Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle--tissue-specific impact on tissue triglyceride content and AKT activation.Endocrinology. 2005 Apr;146(4):2098-106. doi: 10.1210/en.2004-1396. Epub 2004 Dec 23. Endocrinology. 2005. PMID: 15618355
-
Calorie restriction increases muscle mitochondrial biogenesis in healthy humans.PLoS Med. 2007 Mar;4(3):e76. doi: 10.1371/journal.pmed.0040076. PLoS Med. 2007. PMID: 17341128 Free PMC article. Clinical Trial.
-
The influence of dietary fat source on liver and skeletal muscle mitochondrial modifications and lifespan changes in calorie-restricted mice.Biogerontology. 2015 Oct;16(5):655-70. doi: 10.1007/s10522-015-9572-1. Epub 2015 Apr 10. Biogerontology. 2015. PMID: 25860863 Free PMC article. Review.
-
Mitochondrial adaptations to calorie restriction and bariatric surgery in human skeletal muscle: a systematic review with meta-analysis.Metabolism. 2023 Jan;138:155336. doi: 10.1016/j.metabol.2022.155336. Epub 2022 Oct 24. Metabolism. 2023. PMID: 36302454
Cited by
-
Calorie restriction modulates the transcription of genes related to stress response and longevity in human muscle: The CALERIE study.Aging Cell. 2023 Dec;22(12):e13963. doi: 10.1111/acel.13963. Epub 2023 Oct 12. Aging Cell. 2023. PMID: 37823711 Free PMC article. Clinical Trial.
-
Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2.Nutr Rev. 2021 Jan 1;79(1):98-113. doi: 10.1093/nutrit/nuaa085. Nutr Rev. 2021. PMID: 32940695 Free PMC article. Clinical Trial.
-
Metabolic Reprogramming by Reduced Calorie Intake or Pharmacological Caloric Restriction Mimetics for Improved Cancer Immunotherapy.Cancers (Basel). 2021 Mar 12;13(6):1260. doi: 10.3390/cancers13061260. Cancers (Basel). 2021. PMID: 33809187 Free PMC article. Review.
-
Serum factors mediate changes in mitochondrial bioenergetics associated with diet and exercise interventions.Geroscience. 2024 Feb;46(1):349-365. doi: 10.1007/s11357-023-00855-w. Epub 2023 Jun 27. Geroscience. 2024. PMID: 37368157 Free PMC article.
-
Clinical Insights on Caloric Restriction Mimetics for Mitigating Brain Aging and Related Neurodegeneration.Cell Mol Neurobiol. 2024 Oct 16;44(1):67. doi: 10.1007/s10571-024-01493-2. Cell Mol Neurobiol. 2024. PMID: 39412683 Free PMC article. Review.
References
-
- Chabi B, Adhihetty PJ, Ljubicic V, Hood DA. How is mitochondrial biogenesis affected in mitochondrial disease? Med Sci Sports Exerc. 2005;37(12):2102–2110. - PubMed
-
- Nicholls D. Mitochondrial bioenergetics, aging, and aging-related disease. Sci Aging Knowledge Environ. 2002;2002:pe12. - PubMed
-
- Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182(4):321–331. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

