PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation
- PMID: 27779186
- PMCID: PMC5093342
- DOI: 10.1038/ncomms13280
PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation
Erratum in
-
Author Correction: PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation.Nat Commun. 2025 Jul 4;16(1):6158. doi: 10.1038/s41467-025-60997-7. Nat Commun. 2025. PMID: 40615383 Free PMC article. No abstract available.
Abstract
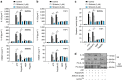
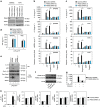
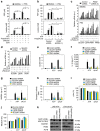
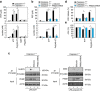
Sepsis, severe sepsis and septic shock are the main cause of mortality in non-cardiac intensive care units. Immunometabolism has been linked to sepsis; however, the precise mechanism by which metabolic reprogramming regulates the inflammatory response is unclear. Here we show that aerobic glycolysis contributes to sepsis by modulating inflammasome activation in macrophages. PKM2-mediated glycolysis promotes inflammasome activation by modulating EIF2AK2 phosphorylation in macrophages. Pharmacological and genetic inhibition of PKM2 or EIF2AK2 attenuates NLRP3 and AIM2 inflammasomes activation, and consequently suppresses the release of IL-1β, IL-18 and HMGB1 by macrophages. Pharmacological inhibition of the PKM2-EIF2AK2 pathway protects mice from lethal endotoxemia and polymicrobial sepsis. Moreover, conditional knockout of PKM2 in myeloid cells protects mice from septic death induced by NLRP3 and AIM2 inflammasome activation. These findings define an important role of PKM2 in immunometabolism and guide future development of therapeutic strategies to treat sepsis.
Conflict of interest statement
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Wang H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

