25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome
- PMID: 27779191
- PMCID: PMC5093305
- DOI: 10.1038/ncomms13129
25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome
Abstract
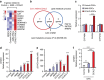
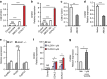
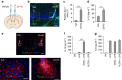
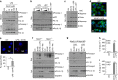
X-linked adrenoleukodystrophy (X-ALD), caused by an ABCD1 mutation, is a progressive neurodegenerative disorder associated with the accumulation of very long-chain fatty acids (VLCFA). Cerebral inflammatory demyelination is the major feature of childhood cerebral ALD (CCALD), the most severe form of ALD, but its underlying mechanism remains poorly understood. Here, we identify the aberrant production of cholesterol 25-hydroxylase (CH25H) and 25-hydroxycholesterol (25-HC) in the cellular context of CCALD based on the analysis of ALD patient-derived induced pluripotent stem cells and ex vivo fibroblasts. Intriguingly, 25-HC, but not VLCFA, promotes robust NLRP3 inflammasome assembly and activation via potassium efflux-, mitochondrial reactive oxygen species (ROS)- and liver X receptor (LXR)-mediated pathways. Furthermore, stereotaxic injection of 25-HC into the corpus callosum of mouse brains induces microglial recruitment, interleukin-1β production, and oligodendrocyte cell death in an NLRP3 inflammasome-dependent manner. Collectively, our results indicate that 25-HC mediates the neuroinflammation of X-ALD via activation of the NLRP3 inflammasome.
Figures

References
-
- Mosser J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993). - PubMed
-
- Powers J. M., Liu Y., Moser A. B. & Moser H. W. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 51, 630–643 (1992). - PubMed
-
- Moser H. W. et al. Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology 31, 1241–1249 (1981). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

