NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity
- PMID: 27779384
- PMCID: PMC5129030
- DOI: 10.1021/jacs.6b08937
NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity
Abstract
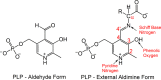
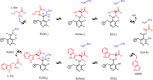
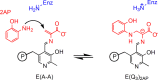
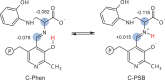
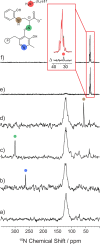
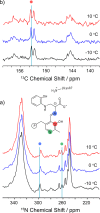
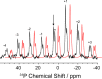
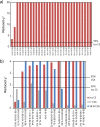
Carbanionic intermediates play a central role in the catalytic transformations of amino acids performed by pyridoxal-5'-phosphate (PLP)-dependent enzymes. Here, we make use of NMR crystallography-the synergistic combination of solid-state nuclear magnetic resonance, X-ray crystallography, and computational chemistry-to interrogate a carbanionic/quinonoid intermediate analogue in the β-subunit active site of the PLP-requiring enzyme tryptophan synthase. The solid-state NMR chemical shifts of the PLP pyridine ring nitrogen and additional sites, coupled with first-principles computational models, allow a detailed model of protonation states for ionizable groups on the cofactor, substrates, and nearby catalytic residues to be established. Most significantly, we find that a deprotonated pyridine nitrogen on PLP precludes formation of a true quinonoid species and that there is an equilibrium between the phenolic and protonated Schiff base tautomeric forms of this intermediate. Natural bond orbital analysis indicates that the latter builds up negative charge at the substrate Cα and positive charge at C4' of the cofactor, consistent with its role as the catalytic tautomer. These findings support the hypothesis that the specificity for β-elimination/replacement versus transamination is dictated in part by the protonation states of ionizable groups on PLP and the reacting substrates and underscore the essential role that NMR crystallography can play in characterizing both chemical structure and dynamics within functioning enzyme active sites.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2109235119. doi: 10.1073/pnas.2109235119. Proc Natl Acad Sci U S A. 2022. PMID: 34996869 Free PMC article.
-
Catalytic roles of βLys87 in tryptophan synthase: (15)N solid state NMR studies.Biochim Biophys Acta. 2015 Sep;1854(9):1194-9. doi: 10.1016/j.bbapap.2015.02.003. Epub 2015 Feb 14. Biochim Biophys Acta. 2015. PMID: 25688830 Free PMC article.
-
X-ray and NMR crystallography in an enzyme active site: the indoline quinonoid intermediate in tryptophan synthase.J Am Chem Soc. 2011 Jan 12;133(1):4-7. doi: 10.1021/ja106555c. Epub 2010 Dec 10. J Am Chem Soc. 2011. PMID: 21142052
-
Stereospecificity of alpha-proton exchange reactions catalysed by pyridoxal-5'-phosphate-dependent enzymes.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):138-42. doi: 10.1016/s1570-9639(03)00080-3. Biochim Biophys Acta. 2003. PMID: 12686123 Review.
-
Tryptophan synthase: a multienzyme complex with an intramolecular tunnel.Chem Rec. 2001;1(2):140-51. doi: 10.1002/tcr.4. Chem Rec. 2001. PMID: 11893063 Review.
Cited by
-
Allosteric regulation of substrate channeling: Salmonella typhimurium tryptophan synthase.Front Mol Biosci. 2022 Sep 12;9:923042. doi: 10.3389/fmolb.2022.923042. eCollection 2022. Front Mol Biosci. 2022. PMID: 36172042 Free PMC article. Review.
-
The interplay of density functional selection and crystal structure for accurate NMR chemical shift predictions.Faraday Discuss. 2025 Jan 8;255(0):119-142. doi: 10.1039/d4fd00072b. Faraday Discuss. 2025. PMID: 39258864
-
Discovery of antimicrobial agent targeting tryptophan synthase.Protein Sci. 2022 Feb;31(2):432-442. doi: 10.1002/pro.4236. Epub 2021 Nov 26. Protein Sci. 2022. PMID: 34767267 Free PMC article.
-
Human serine racemase structure/activity relationship studies provide mechanistic insight and point to position 84 as a hot spot for β-elimination function.J Biol Chem. 2017 Aug 25;292(34):13986-14002. doi: 10.1074/jbc.M117.777904. Epub 2017 Jul 10. J Biol Chem. 2017. PMID: 28696262 Free PMC article.
-
Sedimentation of large, soluble proteins up to 140 kDa for 1H-detected MAS NMR and 13C DNP NMR - practical aspects.J Biomol NMR. 2024 Sep;78(3):179-192. doi: 10.1007/s10858-024-00444-9. Epub 2024 Jun 21. J Biomol NMR. 2024. PMID: 38904893 Free PMC article.
References
-
- Metzler D. E.; Ikawa M.; Snell E. E. J. Am. Chem. Soc. 1954, 76, 648.10.1021/ja01632a004. - DOI
-
- Walsh C.Enzymatic Reaction Mechanisms; W. H. Freeman: San Francisco, CA, 1979.
-
- Jencks W. P.Catalysis in Chemistry and Enzymology; McGraw-Hill: New York, 1969.
-
- Hayashi H. J. Biochem. 1995, 118, 463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

