Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study
- PMID: 27779568
- PMCID: PMC5323337
- DOI: 10.1097/GME.0000000000000756
Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study
Abstract
Objective: The objective of the present study was to compare the efficacy of two forms of menopausal hormone therapy in alleviating vasomotor symptoms, insomnia, and irritability in early postmenopausal women during 4 years.
Methods: A total of 727 women, aged 42 to 58, within 3 years of their final menstrual period, were randomized to receive oral conjugated estrogens (o-CEE) 0.45 mg (n = 230) or transdermal estradiol (t-E2) 50 μg (n = 225; both with micronized progesterone 200 mg for 12 d each mo), or placebos (PBOs; n = 275). Menopausal symptoms were recorded at screening and at 6, 12, 24, 36, and 48 months postrandomization. Differences in proportions of women with symptoms at baseline and at each follow-up time point were compared by treatment arm using exact χ tests in an intent-to-treat analysis. Differences in treatment effect by race/ethnicity and body mass index were tested using generalized linear mixed effects modeling.
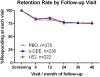
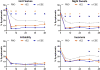
Results: Moderate to severe hot flashes (from 44% at baseline to 28.3% for PBO, 7.4% for t-E2, and 4.2% for o-CEE) and night sweats (from 35% at baseline to 19% for PBO, 5.3% for t-E2, and 4.7% for o-CEE) were reduced significantly by 6 months in women randomized to either active hormone compared with PBO (P < 0.001 for both symptoms), with no significant differences between the active treatment arms. Insomnia and irritability decreased from baseline to 6 months postrandomization in all groups. There was an intermittent reduction in insomnia in both active treatment arms versus PBO, with o-CEE being more effective than PBO at 36 and 48 months (P = 0.002 and 0.05) and t-E2 being more effective than PBO at 48 months (P = 0.004). Neither hormone treatment significantly affected irritability compared with PBO. Symptom relief for active treatment versus PBO was not significantly modified by body mass index or race/ethnicity.
Conclusions: Recently postmenopausal women had similar and substantial reductions in hot flashes and night sweats with lower-than-conventional doses of oral or transdermal estrogen. These reductions were sustained during 4 years. Insomnia was intermittently reduced compared with PBO for both hormone regimens.
Conflict of interest statement
Conflict of Interest disclosures:
Nanette Santoro, MD, reports investigator initiated grant support from Bayer, Inc and stock options in Menogenix, outside the submitted work
Dennis Black, PhD, reports grant and personal fees from Novartis, personal fees from Merck, Amgen, and Eli Lilly, outside the submitted work
Eliot Brinton, MD, reports personal fees from Alexion, Amarin, Amgen, Aralez, Janssen, Kowa, Merck, Regeneron, Sanofi Aventis and Takeda
S Mitchell Harman, MD, reports grants from The Aurora Foundation and Pfizer Pharmaceuticals, non-financial support from Abbott Laboratories and non-financial support from Bayer Healthcare during the conduct of the KEEPS study
Lubna Pal, MD, MSc, reports personal fees from Merck
Roger Lobo M.D. reports consultation fees from Pfizer, Amigen, Teva and grant support from TherapeuticsMD.
Hugh Taylor, MD, PhD, reports grant support from Pfizer through Yale University and personal fees from Pfizer
Erin Wolff MD, PhD, reports being an employee of Celmatix, Inc.
Other coauthors report no disclosures.
Figures
Comment in
-
Low-dose hormone therapy and menopausal symptoms: the ongoing quest for relief.Menopause. 2017 Mar;24(3):235-236. doi: 10.1097/GME.0000000000000831. Menopause. 2017. PMID: 28118299 No abstract available.
References
-
- Multiple authors. Management of menopausal symptoms. Am J Med. 2005;118
-
- Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. Jama. 2005;294:183–93. - PubMed
-
- Barber C, Margolis K, Luepker R. The impact of the Women’s Health Initiative on discontiuation of postmenopausal hormone therapy: the Minnesota Heart Survey. J Womens Health (Lrchmt) 2004;13:975–85. - PubMed
-
- Steingold KA, Laufer L, Chetkowski RJ, et al. Treatment of hot flashes with transdermal estradiol administration. J Clin Endocrinol Metab. 1985;61:627–32. - PubMed
-
- Manson JE, Kaunitz AM. Menopause Management–Getting Clinical Care Back on Track. N Engl J Med. 2016;374:803–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

