Ablation of Pericyte-Like Cells in Lungs by Oropharyngeal Aspiration of Diphtheria Toxin
- PMID: 27779900
- PMCID: PMC5359647
- DOI: 10.1165/rcmb.2016-0083MA
Ablation of Pericyte-Like Cells in Lungs by Oropharyngeal Aspiration of Diphtheria Toxin
Abstract
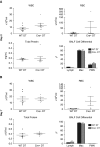
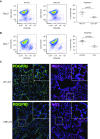
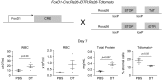
We demonstrated previously that FoxD1-derived cells in the lung are enriched in pericyte-like cells in mouse lung. These cells express the common pericyte markers and are located adjacent to endothelial cells. In this study, we demonstrate the feasibility of administering diphtheria toxin (DT) by oropharyngeal aspiration as an approach to ablating FoxD1-derived cells. We crossed mice expressing Cre-recombinase under the FoxD1 promoter to Rosa26-loxP-STOP-loxP-iDTR mice and generated a bitransgenic line (FoxD1-Cre;Rs26-iDTR) in which FoxD1-derived cells heritably express simian or human diphtheria toxin receptor and are sensitive to DT. We delivered low-dose (0.5 ng/g) and high-dose (1ng/g × 2) to FoxD1-Cre;Rs26-iDTR mice and littermate control mice by oropharyngeal aspiration and evaluated ablation by flow cytometry and immunohistochemistry. FoxD1-Cre mice showed a 40-50% reduction in PDGFRβ+ cells by flow cytometry at Days 2 and 7 after DT administration, with a return of PDGFRβ+ cells at Day 28. Confocal microscopy revealed an observable reduction in pericyte markers. Bronchoalveolar lavage fluid analysis revealed no significant differences in total protein, bronchoalveolar lavage fluid red blood cell, or white blood cell counts at low dose. However, at high-dose DT, there was a proinflammatory effect in the control mice and increased mortality associated with systemic toxicity in Cre+ mice. Low-dose DT reduced lung PDGFRβ+ stromal cells in the FoxD1-Cre;iDTR transgenic model without a differential effect on lung inflammation in DT-sensitive and DT-insensitive animals. Low-dose DT is a viable method for transient lineage-specific stromal cell ablation in the lung that minimizes systemic toxicity.
Keywords: FoxD1; ablation; diphtheria toxin; pericytes; platelet-derived growth factor receptor β.
Figures

References
-
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
-
- Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol Dial Transplant. 2012;27:2149–2155. - PubMed
-
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. - PubMed
-
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Brühl ML, Gärtner F, Khandoga AG, Legate KR, Pless R, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

