The Evolving Neural and Genetic Architecture of Vertebrate Olfaction
- PMID: 27780046
- PMCID: PMC5104188
- DOI: 10.1016/j.cub.2016.09.011
The Evolving Neural and Genetic Architecture of Vertebrate Olfaction
Abstract
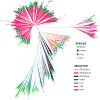
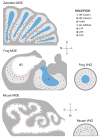
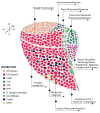
Evolution sculpts the olfactory nervous system in response to the unique sensory challenges facing each species. In vertebrates, dramatic and diverse adaptations to the chemical environment are possible because of the hierarchical structure of the olfactory receptor (OR) gene superfamily: expansion or contraction of OR subfamilies accompanies major changes in habitat and lifestyle; independent selection on OR subfamilies can permit local adaptation or conserved chemical communication; and genetic variation in single OR genes can alter odor percepts and behaviors driven by precise chemical cues. However, this genetic flexibility contrasts with the relatively fixed neural architecture of the vertebrate olfactory system, which requires that new olfactory receptors integrate into segregated and functionally distinct neural pathways. This organization allows evolution to couple critical chemical signals with selectively advantageous responses, but also constrains relationships between olfactory receptors and behavior. The coevolution of the OR repertoire and the olfactory system therefore reveals general principles of how the brain solves specific sensory problems and how it adapts to new ones.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- Wyatt TD. Introduction to Chemical Signaling in Vertebrates and Invertebrates. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): 2014. - PubMed
-
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. - PubMed
-
- Hansson BS, Stensmyr MC. Evolution of insect olfaction. Neuron. 2011;72:698–711. - PubMed
-
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

