Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis
- PMID: 27780265
- PMCID: PMC5079581
- DOI: 10.1371/journal.pone.0165373
Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis
Abstract
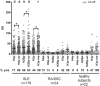
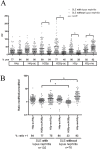
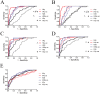
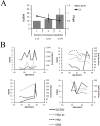
Persistent exposure of the immune system to death cell debris leads to autoantibodies against chromatin in patients with systemic lupus erythematosus (SLE). Deposition of anti-chromatin/chromatin complexes can instigate inflammation in multiple organs including the kidney. Previously we identified specific cell death-associated histone modifications as targets of autoantibodies in SLE. In this study we addressed, in a large cohort of SLE patients and controls, the question whether plasma reactivities with specific histone peptides associated with serology and clinical features. Plasma from SLE patients with and without lupus nephritis, disease controls, and healthy controls, were tested in ELISA with histone H4 peptide acetylated at lysines 8, 12 and 16 (H4pac), H2B peptide acetylated at lysine 12 (H2Bpac), H3 peptide trimethylated at lysine 27 (H3pme), and their unmodified equivalents. SLE patients displayed a higher reactivity with the modified equivalent of each peptide. Reactivity with H4pac showed both a high sensitivity (89%) and specificity (91%) for SLE, while H2Bpac exhibited a high specificity (96%) but lower sensitivity (69%). Reactivity with H3pme appeared not specific for SLE. Anti-H4pac and anti-H2Bpac reactivity demonstrated a high correlation with disease activity. Moreover, patients reacting with multiple modified histone peptides exhibited higher SLEDAI and lower C3 levels. SLE patients with renal involvement showed higher reactivity with H2B/H2Bpac and a more pronounced reactivity with the modified equivalent of H3pme and H2Bpac. In conclusion, reactivity with H4pac and H2Bpac is specific for SLE patients and correlates with disease activity, whereas reactivity with H2Bpac is in particular associated with lupus nephritis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies.Mol Immunol. 2009 Dec;47(2-3):511-6. doi: 10.1016/j.molimm.2009.08.009. Epub 2009 Sep 10. Mol Immunol. 2009. PMID: 19747733
-
The lupus erythematosus cell phenomenon: comparative analysis of antichromatin antibody specificity in lupus erythematosus cell-positive and -negative sera.Arthritis Rheum. 2000 Feb;43(2):420-8. doi: 10.1002/1529-0131(200002)43:2<420::AID-ANR24>3.0.CO;2-Z. Arthritis Rheum. 2000. PMID: 10693884
-
Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus.Arthritis Rheum. 2007 Jun;56(6):1921-33. doi: 10.1002/art.22646. Arthritis Rheum. 2007. PMID: 17530637
-
Clinical application of protein biomarkers in lupus erythematosus and lupus nephritis.Lupus. 2018 Sep;27(10):1582-1590. doi: 10.1177/0961203318773643. Epub 2018 May 3. Lupus. 2018. PMID: 29720035 Review.
-
Paediatric-onset systemic lupus erythematosus.Best Pract Res Clin Rheumatol. 2013 Jun;27(3):351-62. doi: 10.1016/j.berh.2013.07.007. Best Pract Res Clin Rheumatol. 2013. PMID: 24238692 Review.
Cited by
-
Low Levels of IgM and IgA Recognizing Acetylated C1-Inhibitor Peptides Are Associated with Systemic Lupus Erythematosus in Taiwanese Women.Molecules. 2019 Apr 26;24(9):1645. doi: 10.3390/molecules24091645. Molecules. 2019. PMID: 31027344 Free PMC article.
-
A repertoire of 124 potential autoantigens for autoimmune kidney diseases identified by dermatan sulfate affinity enrichment of kidney tissue proteins.PLoS One. 2019 Jun 25;14(6):e0219018. doi: 10.1371/journal.pone.0219018. eCollection 2019. PLoS One. 2019. PMID: 31237920 Free PMC article.
-
B cell profiles, antibody repertoire and reactivity reveal dysregulated responses with autoimmune features in melanoma.Nat Commun. 2023 Jun 8;14(1):3378. doi: 10.1038/s41467-023-39042-y. Nat Commun. 2023. PMID: 37291228 Free PMC article.
-
6-Hydroxydopamine Induces Neurodegeneration in Terminally Differentiated SH-SY5Y Neuroblastoma Cells via Enrichment of the Nucleosomal Degradation Pathway: a Global Proteomics Approach.J Mol Neurosci. 2022 May;72(5):1026-1046. doi: 10.1007/s12031-021-01962-z. Epub 2022 Mar 8. J Mol Neurosci. 2022. PMID: 35258800 Free PMC article.
-
SARS-CoV-2 Antibody Isotypes in Systemic Lupus Erythematosus Patients Prior to Vaccination: Associations With Disease Activity, Antinuclear Antibodies, and Immunomodulatory Drugs During the First Year of the Pandemic.Front Immunol. 2021 Aug 27;12:724047. doi: 10.3389/fimmu.2021.724047. eCollection 2021. Front Immunol. 2021. PMID: 34512651 Free PMC article.
References
-
- Dieker JW, van der Vlag J, Berden JH. Triggers for anti-chromatin autoantibody production in SLE. Lupus. 2002;11: 856–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

