Lessons learned on the design and the conduct of Post-Authorization Safety Studies: review of 3 years of PRAC oversight
- PMID: 27780289
- PMCID: PMC5346857
- DOI: 10.1111/bcp.13165
Lessons learned on the design and the conduct of Post-Authorization Safety Studies: review of 3 years of PRAC oversight
Abstract
Aims: To describe and characterize the first cohort of Post-Authorization Safety Study (PASS) protocols reviewed under the recent European pharmacovigilance legislation.
Methods: A systematic approach was used to compile all publicly available information on PASS protocols and assessments submitted from July 2012 to July 2015 from Pharmacovigilance Risk Assessment Committee (PRAC) minutes, European Medicines Agency (EMA) and European Network of Pharmacovigilance and Pharmacoepidemiology (ENCePP) webpages.
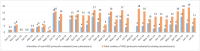
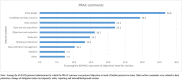
Results: During the study period, 189 different PASS protocols were submitted to the PRAC, half of which were entered in the ENCePP electronic register of post-authorization studies (EU-PAS) by July 2015. Those protocols were assessed during 353 PRAC reviews. The EMA published only 31% of the PRAC feedback, of which the main concerns were study design (37%) and feasibility (30%). Among the 189 PASS, slightly more involved primary data capture (58%). PASS assessing drug utilization mainly leveraged secondary data sources (58%). The majority of the PASS did not include a comparator (65%) and 35% of PASS also evaluated clinical effectiveness endpoints.
Conclusions: To the best of our knowledge this is the first comprehensive review of three years of PASS protocols submitted under the new pharmacovigilance legislation. Our results show that both EMA and PASS sponsors could respectively increase the availability of protocol assessments and documents in the EU-PAS. Protocol content review and the high number of PRAC comments related to methodological issues and feasibility concerns should raise awareness among PASS stakeholders to design more thoughtful studies according to pharmacoepidemiological principles and existing guidelines.
Keywords: ENCePP; PASS; PRAC; new pharmacovigilance legislation; transparency.
© 2016 The British Pharmacological Society.
Figures
References
-
- European Commission . Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. OJ EU 2010; L/348: 74–99 [online]. Available at http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074... (last accessed 10 October 2016).
-
- European Commission . Regulation (EU) No 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. OJ EU 2010; L348: 1–16 [online]. Available at http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0001... (last accessed 10 October 2016).
-
- European Medicines Agency . Pharmacovigilance legislation [online]. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general... (last accessed 10 October 2016).
-
- European Medicines Agency . Guideline on good pharmacovigilance practices (GVP) – Module VIII: Post‐authorisation safety studies (Rev 2). Doc. Ref. EMA/813938/2011 Rev 2; August 2016. [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (last accessed 10 October 2016).
-
- European Commission . Commission Regulation (EC) No 507/2006 of 29 March 2006 on the conditional marketing authorisation for medicinal products for human use falling within the scope of Regulation (EC) No 726/2004 of the European Parliament and of the Council. OJ EU 2006; L/92: 6–9 [online]. Available at http://ec.europa.eu/health/files/eudralex/vol‐1/reg_2006_507/reg_2006_50... (last accessed 3 May 2016).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials