Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation
- PMID: 27780297
- PMCID: PMC5346864
- DOI: 10.1111/bcp.13163
Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation
Abstract
Aim: To evaluate the pharmacokinetics, pharmacodynamics, nasal tolerance and effects on sedation of a highly concentrated aqueous intranasal midazolam formulation (Nazolam) and to compare these to intravenous midazolam.
Methods: In this four-way crossover, double-blind, double-dummy, randomized, placebo-controlled study, 16 subjects received 2.5 mg Nazolam, 5.0 mg Nazolam, 2.5 mg intravenous midazolam or placebo on different occasions. Pharmacokinetics of midazolam and α-hydroxy-midazolam were characterized and related to outcome variables for sedation (saccadic peak velocity, the Bond and Lader visual analogue scale for sedation, the simple reaction time task and the observer's assessment of alertness/sedation). Nasal tolerance was evaluated through subject reporting, and ear, nose and throat examination.
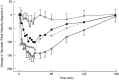
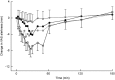
Results: Nazolam bioavailability was 75%. Maximal plasma concentrations of 31 ng ml-1 (CV, 42.3%) were reached after 11 min (2.5 mg Nazolam), and of 66 ng ml-1 (coefficient of variability, 31.5%) after 14 min (5.0 mg Nazolam). Nazolam displayed a significant effect on OAA/S scores. Sedation onset (based on SPV change) occurred 1 ± 0.7 min after administration of 2.5 mg intravenous midazolam, 7 ± 4.4 min after 2.5 mg Nazolam, and 4 ± 1.8 min after 5 mg Nazolam. Sedation duration was 118 ± 95.6 min for 2.5 mg intravenous midazolam, 76 ± 80.4 min for 2.5 mg Nazolam, and 145 ± 104.9 min for 5.0 mg Nazolam. Nazolam did not lead to nasal mucosa damage.
Conclusions: This study demonstrates the nasal tolerance, safety and efficacy of Nazolam. When considering the preparation time needed for obtaining venous access, conscious sedation can be achieved in the same time span as needed for intravenous midazolam. Nazolam may offer important advantages in conscious sedation.
Keywords: conscious sedation; intranasal; midazolam; pharmacodynamics; pharmacokinetics.
© 2016 The British Pharmacological Society.
Figures



References
-
- Midazolam FDA label. Available at http://www.fda.gov/ohrms/dockets/dailys/01/Mar01/032101/cp00001_exhibit_... (last accessed 30 May 2016).
-
- Pitetti R, Davis PJ, Redlinger R, White J, Wiener E, Calhoun KH. Effect on hospital‐wide sedation practices after implementation of the 2001 JCAHO procedural sedation and analgesia guidelines. Arch Pediatr Adolesc Med 2006; 160: 211–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

