Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA
- PMID: 27780818
- PMCID: PMC5283882
- DOI: 10.1152/ajpendo.00116.2016
Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA
Abstract
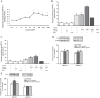
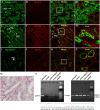
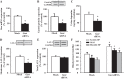
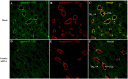
Gastrin is a peptide hormone that is involved in the regulation of sodium balance and blood pressure. Dopamine, which is also involved in the regulation of sodium balance and blood pressure, directly or indirectly interacts with other blood pressure-regulating hormones, including gastrin. This study aimed to determine the mechanisms of the interaction between gastrin and dopamine and tested the hypothesis that gastrin produced in the kidney increases renal dopamine production to keep blood pressure within the normal range. We show that in human and mouse renal proximal tubule cells (hRPTCs and mRPTCs, respectively), gastrin stimulates renal dopamine production by increasing the cellular uptake of l-DOPA via the l-type amino acid transporter (LAT) at the plasma membrane. The uptake of l-DOPA in RPTCs from C57Bl/6J mice is lower than in RPTCs from normotensive humans. l-DOPA uptake in renal cortical slices is also lower in salt-sensitive C57Bl/6J than in salt-resistant BALB/c mice. The deficient renal cortical uptake of l-DOPA in C57Bl/6J mice may be due to decreased LAT-1 activity that is related to its decreased expression at the plasma membrane, relative to BALB/c mice. We also show that renal-selective silencing of Gast by the renal subcapsular injection of Gast siRNA in BALB/c mice decreases renal dopamine production and increases blood pressure. These results highlight the importance of renal gastrin in stimulating renal dopamine production, which may give a new perspective in the prevention and treatment of hypertension.
Keywords: dopamine; gastrin; hypertension; l-DOPA; l-type amino acid transporter.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Armando I, Nowicki S, Aguirre J, Barontini M. A decreased tubular uptake of dopa results in defective renal dopamine production in aged rats. Am J Physiol Renal Physiol 268: F1087–F1092, 1995. - PubMed
-
- Carranza A, Nowicki S, Barontini M, Armando I. L-Dopa uptake and dopamine production in proximal tubular cells are regulated by β(2)-adrenergic receptors. Am J Physiol Renal Physiol 279: F77–F83, 2000. - PubMed
-
- Carranza A, Karabatas L, Barontini M, Armando I. Decreased tubular uptake of L-3,4-dihydroxyphenylalanine in streptozotocin-induced diabetic rats. Horm Res 55: 282–287, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

