Protein 4.1R Influences Myogenin Protein Stability and Skeletal Muscle Differentiation
- PMID: 27780863
- PMCID: PMC5207257
- DOI: 10.1074/jbc.M116.761296
Protein 4.1R Influences Myogenin Protein Stability and Skeletal Muscle Differentiation
Abstract
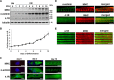
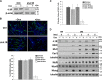
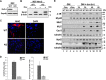
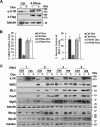
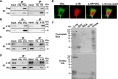
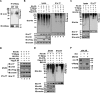
Protein 4.1R (4.1R) isoforms are expressed in both cardiac and skeletal muscle. 4.1R is a component of the contractile apparatus. It is also associated with dystrophin at the sarcolemma in skeletal myofibers. However, the expression and function of 4.1R during myogenesis have not been characterized. We now report that 4.1R expression increases during C2C12 myoblast differentiation into myotubes. Depletion of 4.1R impairs skeletal muscle differentiation and is accompanied by a decrease in the levels of myosin heavy and light chains and caveolin-3. Furthermore, the expression of myogenin at the protein, but not mRNA, level is drastically decreased in 4.1R knockdown myocytes. Similar results were obtained using MyoD-induced differentiation of 4.1R-/- mouse embryonic fibroblast cells. von Hippel-Lindau (VHL) protein is known to destabilize myogenin via the ubiquitin-proteasome pathway. We show that 4.1R associates with VHL and, when overexpressed, reverses myogenin ubiquitination and stability. This suggests that 4.1R may influence myogenesis by preventing VHL-mediated myogenin degradation. Together, our results define a novel biological function for 4.1R in muscle differentiation and provide a molecular mechanism by which 4.1R promotes myogenic differentiation.
Keywords: E3 ubiquitin ligase; myogenesis; myogenin; protein 4.1R; protein degradation; protein stability; ubiquitylation (ubiquitination); von Hippel Lindau.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
EGLN3 prolyl hydroxylase regulates skeletal muscle differentiation and myogenin protein stability.J Biol Chem. 2007 Apr 27;282(17):12410-8. doi: 10.1074/jbc.M608748200. Epub 2007 Mar 6. J Biol Chem. 2007. PMID: 17344222
-
Magnesium deficiency up-regulates Myod expression in rat skeletal muscle and C2C12 myogenic cells.Cell Biochem Funct. 2011 Oct;29(7):577-81. doi: 10.1002/cbf.1790. Epub 2011 Aug 19. Cell Biochem Funct. 2011. PMID: 21858842
-
Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin.Exp Cell Res. 2003 Jun 10;286(2):263-75. doi: 10.1016/s0014-4827(03)00074-0. Exp Cell Res. 2003. PMID: 12749855
-
[Effects of mechanical stretch with variant frequencies on alignment and differentiation of multilayer myotubes cultured in vitro].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012 Jun;26(6):735-42. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012. PMID: 22792775 Chinese.
-
Regulation of Skeletal Myoblast Differentiation by Drebrin.Adv Exp Med Biol. 2017;1006:361-373. doi: 10.1007/978-4-431-56550-5_22. Adv Exp Med Biol. 2017. PMID: 28865032 Review.
Cited by
-
Epithelial-specific isoforms of protein 4.1R promote adherens junction assembly in maturing epithelia.J Biol Chem. 2020 Jan 3;295(1):191-211. doi: 10.1074/jbc.RA119.009650. Epub 2019 Nov 27. J Biol Chem. 2020. PMID: 31776189 Free PMC article.
-
Differential analysis of ubiquitin-proteomics in skeletal muscle of Duroc pigs and Tibetan fragrant pigs.Front Vet Sci. 2024 Aug 30;11:1455338. doi: 10.3389/fvets.2024.1455338. eCollection 2024. Front Vet Sci. 2024. PMID: 39280835 Free PMC article.
-
Role of Actin-Binding Proteins in Skeletal Myogenesis.Cells. 2023 Oct 25;12(21):2523. doi: 10.3390/cells12212523. Cells. 2023. PMID: 37947600 Free PMC article. Review.
-
Thick Filament Protein Network, Functions, and Disease Association.Compr Physiol. 2018 Mar 13;8(2):631-709. doi: 10.1002/cphy.c170023. Compr Physiol. 2018. PMID: 29687901 Free PMC article. Review.
-
Cytoskeletal Protein 4.1R in Health and Diseases.Biomolecules. 2024 Feb 11;14(2):214. doi: 10.3390/biom14020214. Biomolecules. 2024. PMID: 38397451 Free PMC article. Review.
References
-
- Lallena M. J., Martínez C., Valcárcel J., and Correas I. (1998) Functional association of nuclear protein 4.1 with pre-mRNA splicing factors. J. Cell Sci. 111, 1963–1971 - PubMed
-
- Mattagajasingh S. N., Huang S. C., Hartenstein J. S., and Benz E. J. Jr. (2000) Characterization of the interaction between protein 4.1R and ZO-2: a possible link between the tight junction and the actin cytoskeleton. J. Biol. Chem. 275, 30573–30585 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

