The capillary bed offers the largest hemodynamic resistance to the cortical blood supply
- PMID: 27780904
- PMCID: PMC5363755
- DOI: 10.1177/0271678X16671146
The capillary bed offers the largest hemodynamic resistance to the cortical blood supply
Abstract
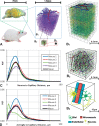
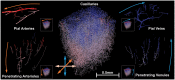
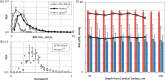
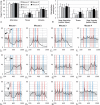
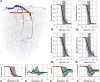
The cortical angioarchitecture is a key factor in controlling cerebral blood flow and oxygen metabolism. Difficulties in imaging the complex microanatomy of the cortex have so far restricted insight about blood flow distribution in the microcirculation. A new methodology combining advanced microscopy data with large scale hemodynamic simulations enabled us to quantify the effect of the angioarchitecture on the cerebral microcirculation. High-resolution images of the mouse primary somatosensory cortex were input into with a comprehensive computational model of cerebral perfusion and oxygen supply ranging from the pial vessels to individual brain cells. Simulations of blood flow, hematocrit and oxygen tension show that the wide variation of hemodynamic states in the tortuous, randomly organized capillary bed is responsible for relatively uniform cortical tissue perfusion and oxygenation. Computational analysis of microcirculatory blood flow and pressure drops further indicates that the capillary bed, including capillaries adjacent to feeding arterioles (d < 10 µm), are the largest contributors to hydraulic resistance.
Keywords: Mathematical modelling; blood–brain barrier; cerebral blood flow; cerebral hemodynamics; microcirculation.
© The Author(s) 2016.
Figures



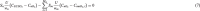

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

