Chondrogenic Potency Analyses of Donor-Matched Chondrocytes and Mesenchymal Stem Cells Derived from Bone Marrow, Infrapatellar Fat Pad, and Subcutaneous Fat
- PMID: 27781068
- PMCID: PMC5066011
- DOI: 10.1155/2016/6969726
Chondrogenic Potency Analyses of Donor-Matched Chondrocytes and Mesenchymal Stem Cells Derived from Bone Marrow, Infrapatellar Fat Pad, and Subcutaneous Fat
Abstract
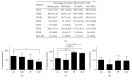
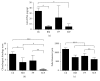
Autologous chondrocyte implantation (ACI) is a cell-based therapy that has been used clinically for over 20 years to treat cartilage injuries more efficiently in order to negate or delay the need for joint replacement surgery. In this time, very little has changed in the ACI procedure, but now many centres are considering or using alternative cell sources for cartilage repair, in particular mesenchymal stem cells (MSCs). In this study, we have tested the chondrogenic potential of donor-matched MSCs derived from bone marrow (BM), infrapatellar fat pad (FP), and subcutaneous fat (SCF), compared to chondrocytes. We have confirmed that there is a chondrogenic potency hierarchy ranging across these cell types, with the most potent being chondrocytes, followed by FP-MSCs, BM-MSCs, and lastly SCF-MSCs. We have also examined gene expression and surface marker profiles in a predictive model to identify cells with enhanced chondrogenic potential. In doing so, we have shown that Sox-9, Alk-1, and Coll X expressions, as well as immunopositivity for CD49c and CD39, have predictive value for all of the cell types tested in indicating chondrogenic potency. The findings from this study have significant clinical implications for the refinement and development of novel cell-based cartilage repair strategies.
Figures


References
-
- Bhosale A. M., Kuiper J. H., Johnson W. E. B., Harrison P. E., Richardson J. B. Midterm to long-term longitudinal outcome of autologous chondrocyte implantation in the knee joint: a multilevel analysis. The American Journal of Sports Medicine. 2009;37(supplement 1):131S–138S. - PubMed
-
- Saris D. B. F., Vanlauwe J., Victor J., et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. The American Journal of Sports Medicine. 2009;37(supplement 1):10S–19S. - PubMed
-
- McCarthy H. S., Richardson J. B., Parker J. C., Roberts S. Evaluating joint morbidity after chondral harvest for autologous chondrocyte implantation (ACI): a study of ACI-treated ankles and hips with a knee chondral harvest. Cartilage. 2016;7(1):7–15. doi: 10.1177/1947603515607963. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

