Seeking for Non-Zinc-Binding MMP-2 Inhibitors: Synthesis, Biological Evaluation and Molecular Modelling Studies
- PMID: 27782083
- PMCID: PMC5085792
- DOI: 10.3390/ijms17101768
Seeking for Non-Zinc-Binding MMP-2 Inhibitors: Synthesis, Biological Evaluation and Molecular Modelling Studies
Abstract
Matrix metalloproteinases (MMPs) are an important family of zinc-containing enzymes with a central role in many physiological and pathological processes. Although several MMP inhibitors have been synthesized over the years, none reached the market because of off-target effects, due to the presence of a zinc binding group in the inhibitor structure. To overcome this problem non-zinc-binding inhibitors (NZIs) have been recently designed. In a previous article, a virtual screening campaign identified some hydroxynaphtyridine and hydroxyquinoline as MMP-2 non-zinc-binding inhibitors. In the present work, simplified analogues of previously-identified hits have been synthesized and tested in enzyme inhibition assays. Docking and molecular dynamics studies were carried out to rationalize the activity data.
Keywords: MMPs; docking; inhibitors; isoquinoline; molecular dynamics; non-zinc-binding group; quinoline; ureas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



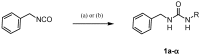



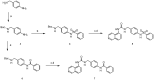


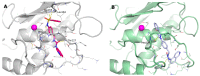
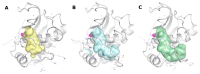

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

