AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes
- PMID: 27782132
- PMCID: PMC5095183
- DOI: 10.1038/ncomms13245
AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes
Abstract
Transmembrane transport of plant hormones is required for plant growth and development. Despite reports of a number of proteins that can transport the plant hormone gibberellin (GA), the mechanistic basis for GA transport and the identities of the transporters involved remain incomplete. Here, we provide evidence that Arabidopsis SWEET proteins, AtSWEET13 and AtSWEET14, which are members of a family that had previously been linked to sugar transport, are able to mediate cellular GA uptake when expressed in yeast and oocytes. A double sweet13 sweet14 mutant has a defect in anther dehiscence and this phenotype can be reversed by exogenous GA treatment. In addition, sweet13 sweet14 exhibits altered long distant transport of exogenously applied GA and altered responses to GA during germination and seedling stages. These results suggest that AtSWEET13 and AtSWEET14 may be involved in modulating GA response in Arabidopsis.
Figures

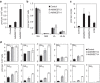


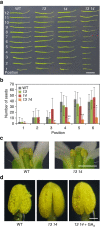
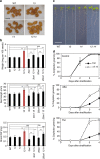
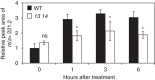
References
-
- Yazaki K. Transporters of secondary metabolites. Curr. Opin. Plant Biol. 8, 301–307 (2005). - PubMed
-
- Petrasek J. & Friml J. Auxin transport routes in plant development. Development 136, 2675–2688 (2009). - PubMed
-
- Blakeslee J. J., Peer W. A. & Murphy A. S. Auxin transport. Curr. Opin. Plant. Biol. 8, 494–500 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

