Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense
- PMID: 27782325
- PMCID: PMC5427221
- DOI: 10.1111/imr.12500
Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense
Abstract
As a preformed defense system, complement faces a delicate challenge in providing an immediate, forceful response to pathogens even at first encounter, while sparing host cells in the process. For this purpose, it engages a tightly regulated network of plasma proteins, cell surface receptors, and regulators. Complement component C3 plays a particularly versatile role in this process by keeping the cascade alert, acting as a point of convergence of activation pathways, fueling the amplification of the complement response, exerting direct effector functions, and helping to coordinate downstream immune responses. In recent years, it has become evident that nature engages the power of C3 not only to clear pathogens but also for a variety of homeostatic processes ranging from tissue regeneration and synapse pruning to clearing debris and controlling tumor cell progression. At the same time, its central position in immune surveillance makes C3 a target for microbial immune evasion and, if improperly engaged, a trigger point for various clinical conditions. In our review, we look at the versatile roles and evolutionary journey of C3, discuss new insights into the molecular basis for C3 function, provide examples of disease involvement, and summarize the emerging potential of C3 as a therapeutic target.
Keywords: compstatin; convertase; immune evasion; inflammation; therapeutics.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
D. R. and J. D. L. are co-inventors of patents and/or patent applications describing complement inhibitors and their clinical use. J. D. L. is the founder of Amyndas Pharmaceuticals.
Figures






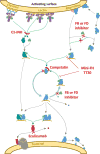
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

