Properdin: a tightly regulated critical inflammatory modulator
- PMID: 27782331
- PMCID: PMC5096056
- DOI: 10.1111/imr.12466
Properdin: a tightly regulated critical inflammatory modulator
Abstract
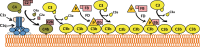
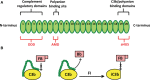
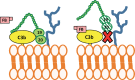
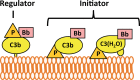
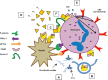
The complement alternative pathway is a powerful arm of the innate immune system that enhances diverse inflammatory responses in the human host. Key to the effects of the alternative pathway is properdin, a serum glycoprotein that can both initiate and positively regulate alternative pathway activity. Properdin is produced by many different leukocyte subsets and circulates as cyclic oligomers of monomeric subunits. While the formation of non-physiological aggregates in purified properdin preparations and the presence of potential properdin inhibitors in serum have complicated studies of its function, properdin has, regardless, emerged as a key player in various inflammatory disease models. Here, we review basic properdin biology, emphasizing the major hurdles that have complicated the interpretation of results from properdin-centered studies. In addition, we elaborate on an emerging role for properdin in thromboinflammation and discuss the potential utility of properdin inhibitors as long-term therapeutic options to treat diseases marked by increased formation of platelet/granulocyte aggregates. Finally, we describe the interplay between properdin and the alternative pathway negative regulator, Factor H, and how aiming to understand these interactions can provide scientists with the most effective ways to manipulate alternative pathway activation in complex systems.
Keywords: complement; factor H; inflammation; properdin.
© 2016 The Authors. Immunological Reviews Published by John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

