Pericyte Seeded Dual Peptide Scaffold with Improved Endothelialization for Vascular Graft Tissue Engineering
- PMID: 27782370
- PMCID: PMC5405341
- DOI: 10.1002/adhm.201600699
Pericyte Seeded Dual Peptide Scaffold with Improved Endothelialization for Vascular Graft Tissue Engineering
Abstract
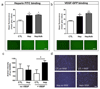
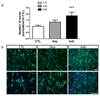
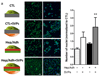
The development of synthetic vascular grafts for coronary artery bypass is challenged by insufficient endothelialization, which increases the risk of thrombosis, and the lack of native cellular constituents, which favors pathological remodeling. Here, a bifunctional electrospun poly(ε-caprolactone) (PCL) scaffold with potential for synthetic vascular graft applications is presented. This scaffold incorporates two tethered peptides: the osteopontin-derived peptide (Adh) on the "luminal" side and a heparin-binding peptide (Hep) on the "abluminal" side. Additionally, the "abluminal" side of the scaffold is seeded with saphenous vein-derived pericytes (SVPs) as a source of proangiogenic growth factors. The Adh peptide significantly increases endothelial cell adhesion, while the Hep peptide promotes accumulation of vascular endothelial growth factor secreted by SVPs. SVPs increase endothelial migration both in a transwell assay and a modified scratch assay performed on the PCL scaffold. Seeding of SVPs on the "abluminal"/Hep side of the scaffold further increases endothelial cell density, indicating a combinatory effect of the peptides and pericytes. Finally, SVP-seeded scaffolds are preserved by freezing in a xeno-free medium, maintaining good cell viability and function. In conclusion, this engineered scaffold combines patient-derived pericytes and spatially organized functionalities, which synergistically increase endothelial cell density and growth factor retention.
Keywords: biofunctionalization; electrospinning; endothelialization; pericytes; tissue engineered vascular graft.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- SP/12/7/29572/BHF_/British Heart Foundation/United Kingdom
- FS/16/9/32012/BHF_/British Heart Foundation/United Kingdom
- RM/13/2/30158/BHF_/British Heart Foundation/United Kingdom
- PG/10/81/28606/BHF_/British Heart Foundation/United Kingdom
- MR/K026682/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

