Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: the Princess Margaret IMPACT/COMPACT trial
- PMID: 27782854
- PMCID: PMC5078968
- DOI: 10.1186/s13073-016-0364-2
Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: the Princess Margaret IMPACT/COMPACT trial
Abstract
Background: The clinical utility of molecular profiling of tumor tissue to guide treatment of patients with advanced solid tumors is unknown. Our objectives were to evaluate the frequency of genomic alterations, clinical "actionability" of somatic variants, enrollment in mutation-targeted or other clinical trials, and outcome of molecular profiling for advanced solid tumor patients at the Princess Margaret Cancer Centre (PM).
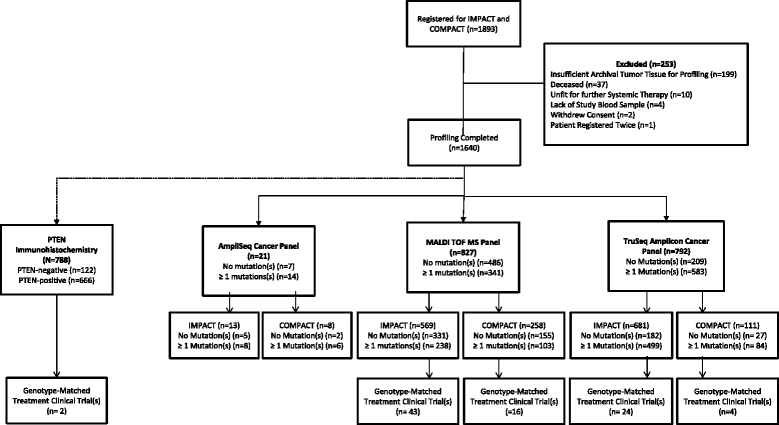
Methods: Patients with advanced solid tumors aged ≥18 years, good performance status, and archival tumor tissue available were prospectively consented. DNA from archival formalin-fixed paraffin-embedded tumor tissue was tested using a MALDI-TOF MS hotspot panel or a targeted next generation sequencing (NGS) panel. Somatic variants were classified according to clinical actionability and an annotated report included in the electronic medical record. Oncologists were provided with summary tables of their patients' molecular profiling results and available mutation-specific clinical trials. Enrolment in genotype-matched versus genotype-unmatched clinical trials following release of profiling results and response by RECIST v1.1 criteria were evaluated.
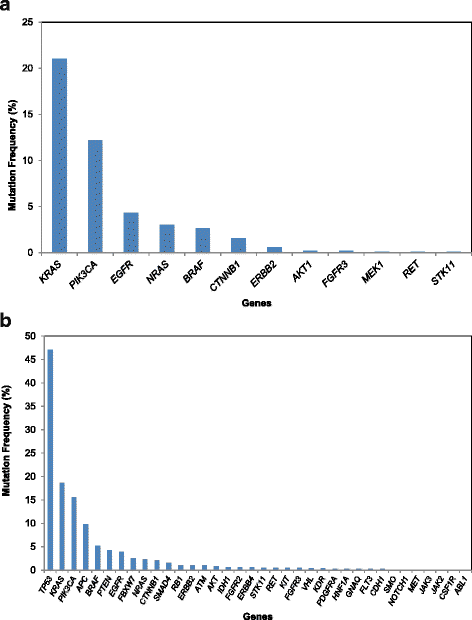
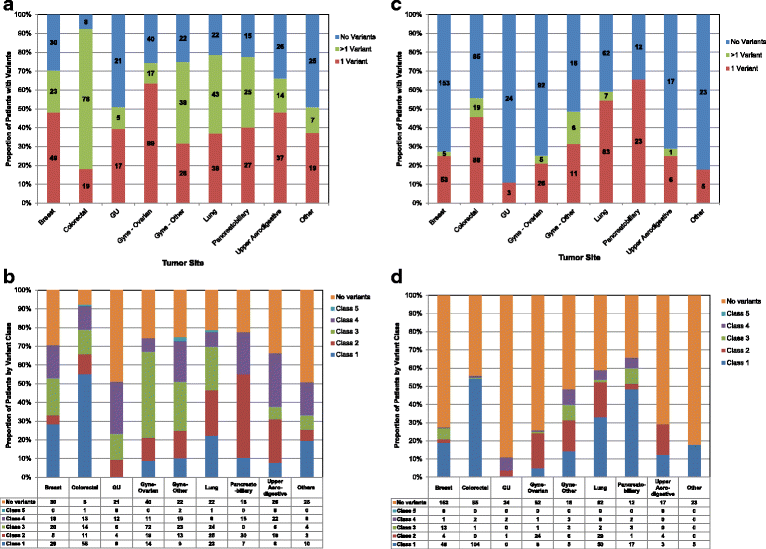
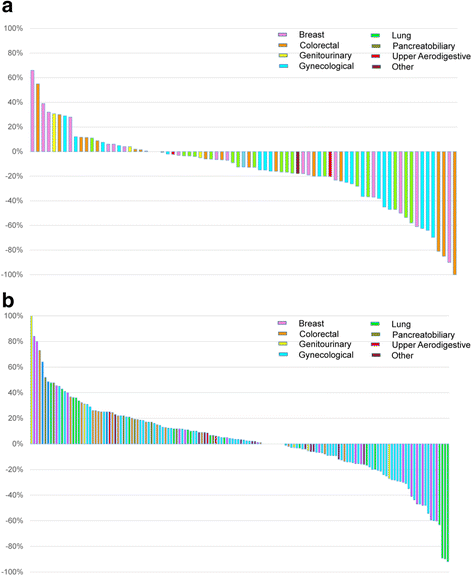
Results: From March 2012 to July 2014, 1893 patients were enrolled and 1640 tested. After a median follow-up of 18 months, 245 patients (15 %) who were tested were subsequently treated on 277 therapeutic clinical trials, including 84 patients (5 %) on 89 genotype-matched trials. The overall response rate was higher in patients treated on genotype-matched trials (19 %) compared with genotype-unmatched trials (9 %; p < 0.026). In a multi-variable model, trial matching by genotype (p = 0.021) and female gender (p = 0.034) were the only factors associated with increased likelihood of treatment response.
Conclusions: Few advanced solid tumor patients enrolled in a prospective institutional molecular profiling trial were treated subsequently on genotype-matched therapeutic trials. In this non-randomized comparison, genotype-enrichment of early phase clinical trials was associated with an increased objective tumor response rate.
Trial registration: NCT01505400 (date of registration 4 January 2012).
Keywords: Clinical trials; DNA sequencing; Molecular profiling; Precision medicine; Solid tumors.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

