Dissecting the Heterogeneity of Circulating Tumor Cells in Metastatic Breast Cancer: Going Far Beyond the Needle in the Haystack
- PMID: 27783057
- PMCID: PMC5085799
- DOI: 10.3390/ijms17101775
Dissecting the Heterogeneity of Circulating Tumor Cells in Metastatic Breast Cancer: Going Far Beyond the Needle in the Haystack
Abstract
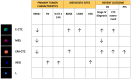
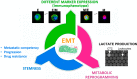
Although the enumeration of circulating tumor cells (CTC) defined as expressing both epithelial cell adhesion molecule and cytokeratins (EpCAM⁺/CK⁺) can predict prognosis and response to therapy in metastatic breast, colon and prostate cancer, its clinical utility (i.e., the ability to improve patient outcome by guiding therapy) has not yet been proven in clinical trials. Therefore, scientists are now focusing on the molecular characterization of CTC as a way to explore its possible use as a "surrogate" of tumor tissues to non-invasively assess the genomic landscape of the cancer and its evolution during treatment. Additionally, evidences confirm the existence of CTC in epithelial-to-mesenchymal transition (EMT) characterized by a variable loss of epithelial markers. Since the EMT process can originate cells with enhanced invasiveness, stemness and drug-resistance, the enumeration and characterization of this population, perhaps the one truly responsible of tumor recurrence and progression, could be more clinically useful. For these reasons, several devices able to capture CTC independently from the expression of epithelial markers have been developed. In this review, we will describe the types of heterogeneity so far identified and the key role played by the epithelial-to-mesenchymal transition in driving CTC heterogeneity. The clinical relevance of detecting CTC-heterogeneity will be discussed as well.
Keywords: circulating tumor cells; epithelial-to-mesenchymal transition; metabolism; metastatic breast cancer; spatial and temporal heterogeneity; stemness.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
-
- Siu L.L. Accounting for tumor heterogeneity in the development of predictive biomarkers. Clin. Adv. Hematol. Oncol. 2013;11:312–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

