Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms
- PMID: 27783860
- PMCID: PMC5242202
- DOI: 10.1002/cphy.c160002
Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms
Abstract
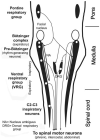
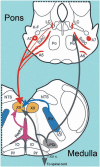
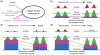
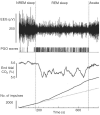
Upper airway muscles subserve many essential for survival orofacial behaviors, including their important role as accessory respiratory muscles. In the face of certain predisposition of craniofacial anatomy, both tonic and phasic inspiratory activation of upper airway muscles is necessary to protect the upper airway against collapse. This protective action is adequate during wakefulness, but fails during sleep which results in recurrent episodes of hypopneas and apneas, a condition known as the obstructive sleep apnea syndrome (OSA). Although OSA is almost exclusively a human disorder, animal models help unveil the basic principles governing the impact of sleep on breathing and upper airway muscle activity. This article discusses the neuroanatomy, neurochemistry, and neurophysiology of the different neuronal systems whose activity changes with sleep-wake states, such as the noradrenergic, serotonergic, cholinergic, orexinergic, histaminergic, GABAergic and glycinergic, and their impact on central respiratory neurons and upper airway motoneurons. Observations of the interactions between sleep-wake states and upper airway muscles in healthy humans and OSA patients are related to findings from animal models with normal upper airway, and various animal models of OSA, including the chronic-intermittent hypoxia model. Using a framework of upper airway motoneurons being under concurrent influence of central respiratory, reflex and state-dependent inputs, different neurotransmitters, and neuropeptides are considered as either causing a sleep-dependent withdrawal of excitation from motoneurons or mediating an active, sleep-related inhibition of motoneurons. Information about the neurochemistry of state-dependent control of upper airway muscles accumulated to date reveals fundamental principles and may help understand and treat OSA. © 2016 American Physiological Society. Compr Physiol 6:1801-1850, 2016.
Copyright © 2016 John Wiley & Sons, Inc.
Figures












References
-
- Adachi M, Nonaka S, Katada A, Arakawa T, Ota R, Harada H, Takakusaki K, Harabuchi Y. Carbachol injection into the pontine reticular formation depresses laryngeal muscle activities and airway reflexes in decerebrate cats. Neurosci Res. 2010;67:40–50. - PubMed
-
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve α2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. - PubMed
-
- Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: Receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600:93–103. - PubMed
-
- Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain wiring versus volume transmission. Neuroscience. 1995;69:711–726. - PubMed
-
- Ahmed WA, Tsutsumi M, Nakata S, Mori T, Nishimura Y, Fujisawa T, Kato I, Nakashima M, Kurahashi H, Suzuki K. A functional variation in the hypocretin neuropeptide precursor gene may be associated with obstructive sleep apnea syndrome in Japan. Laryngoscope. 2012;122:925–929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

