PD-L2 negatively regulates Th1-mediated immunopathology during Fasciola hepatica infection
- PMID: 27783986
- PMCID: PMC5363616
- DOI: 10.18632/oncotarget.12790
PD-L2 negatively regulates Th1-mediated immunopathology during Fasciola hepatica infection
Abstract
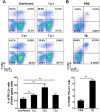
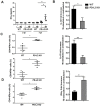
Macrophage plasticity is critical for controlling inflammation including those produced by helminth infections, where alternatively activated macrophages (AAM) are accumulated in tissues. AAM expressing the co-inhibitory molecule programmed death ligand 2 (PD-L2), which is capable of binding programmed death 1 (PD-1) expressed on activated T cells, have been demonstrated in different parasitic infections. However, the role of PD-L2 during F. hepatica infection has not yet been explored. We observed that F. hepatica infection or a F. hepatica total extract (TE) injection increased the expression of PD-L2 on peritoneal macrophages. In addition, the absence of PD-L2 expression correlated with an increase in susceptibility to F. hepatica infection, as evidenced by the shorter survival and increased liver damage observed in PD-L2 deficient (KO) mice. We assessed the contribution of the PD-L2 pathway to Th2 polarization during this infection, and found that the absence of PD-L2 caused a diminished Th2 type cytokine production by TE stimulated splenocytes from PD-L2 KO infected compared with WT mice. Besides, splenocytes and intrahepatic leukocytes from infected PD-L2 KO mice showed higher levels of IFN-γ than those from WT mice. Arginase expression and activity and IL-10 production were reduced in macrophages from PD-L2 KO mice compared to those from WT mice, revealing a strong correlation between PD-L2 expression and AAM polarization. Taken together, our data indicate that PD-L2 expression in macrophages is critical for AAM induction and the maintenance of an optimal balance between the Th1- and Th2-type immune responses to assure host survival during F. hepatica infection.
Keywords: F. hepatica; PD-L2; Th1 response; infection; macrophage.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


Similar articles
-
Immunological interactions between 2 common pathogens, Th1-inducing protozoan Toxoplasma gondii and the Th2-inducing helminth Fasciola hepatica.PLoS One. 2009 May 25;4(5):e5692. doi: 10.1371/journal.pone.0005692. PLoS One. 2009. PMID: 19478853 Free PMC article.
-
Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses.J Immunol. 2009 Aug 1;183(3):1577-86. doi: 10.4049/jimmunol.0803803. Epub 2009 Jul 8. J Immunol. 2009. PMID: 19587018
-
The absence of MyD88 has no effect on the induction of alternatively activated macrophage during Fasciola hepatica infection.BMC Immunol. 2011 Nov 11;12:63. doi: 10.1186/1471-2172-12-63. BMC Immunol. 2011. Retraction in: BMC Immunol. 2012 Jan 16;13:3. doi: 10.1186/1471-2172-13-3. PMID: 22074389 Free PMC article. Retracted.
-
Fasciola hepatica excretory-secretory products induce CD4+T cell anergy via selective up-regulation of PD-L2 expression on macrophages in a Dectin-1 dependent way.Immunobiology. 2015 Jul;220(7):934-9. doi: 10.1016/j.imbio.2015.02.001. Epub 2015 Feb 25. Immunobiology. 2015. PMID: 25758714
-
Fasciola hepatica, TGF-β and host mimicry: the enemy within.Curr Opin Microbiol. 2018 Dec;46:80-85. doi: 10.1016/j.mib.2018.09.002. Epub 2018 Oct 11. Curr Opin Microbiol. 2018. PMID: 30317150 Review.
Cited by
-
Trichinella spiralis Infection Mitigates Collagen-Induced Arthritis via Programmed Death 1-Mediated Immunomodulation.Front Immunol. 2018 Jul 26;9:1566. doi: 10.3389/fimmu.2018.01566. eCollection 2018. Front Immunol. 2018. PMID: 30093899 Free PMC article.
-
Neglected Agent Eminent Disease: Linking Human Helminthic Infection, Inflammation, and Malignancy.Front Cell Infect Microbiol. 2019 Dec 6;9:402. doi: 10.3389/fcimb.2019.00402. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31867284 Free PMC article. Review.
-
Both host and parasite non-coding RNAs co-ordinate the regulation of macrophage gene expression to reduce pro-inflammatory immune responses and promote tissue repair pathways during infection with fasciola hepatica.RNA Biol. 2024 Jan;21(1):62-77. doi: 10.1080/15476286.2024.2408706. Epub 2024 Sep 30. RNA Biol. 2024. PMID: 39344634 Free PMC article.
-
Exploring the role of macrophages in determining the pathogenesis of liver fluke infection.Parasitology. 2022 Sep;149(10):1364-1373. doi: 10.1017/S0031182022000749. Epub 2022 May 27. Parasitology. 2022. PMID: 35621040 Free PMC article. Review.
-
Expression of Programmed Death-Ligand 1 by Human Colonic CD90+ Stromal Cells Differs Between Ulcerative Colitis and Crohn's Disease and Determines Their Capacity to Suppress Th1 Cells.Front Immunol. 2018 May 30;9:1125. doi: 10.3389/fimmu.2018.01125. eCollection 2018. Front Immunol. 2018. PMID: 29910803 Free PMC article.
References
-
- Cuervo PF, Cataldo SD, Fantozzi MC, Deis E, Isenrath GD, Viberti G, Artigas P, Peixoto R, Valero MA, Sierra RM, Mas-Coma S. Liver fluke (Fasciola hepatica) naturally infecting introduced European brown hare (Lepus europaeus) in northern Patagonia: phenotype, prevalence and potential risk. Acta Parasitol. 2015;60:536–543. - PubMed
-
- Marcos LA, Yi P, Machicado A, Andrade R, Samalvides F, Sanchez J, Terashima A. Hepatic fibrosis and Fasciola hepatica infection in cattle. J Helminthol. 2007;81:381–386. - PubMed
-
- Flynn RJ, Mulcahy G, Welsh M, Cassidy JP, Corbett D, Milligan C, Andersen P, Strain S, McNair J. Co-Infection of cattle with Fasciola hepatica and Mycobacterium bovis- immunological consequences. Transbound Emerg Dis. 2009;56:269–274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

