Deubiquitinase USP9X deubiquitinates β-catenin and promotes high grade glioma cell growth
- PMID: 27783990
- PMCID: PMC5346732
- DOI: 10.18632/oncotarget.12819
Deubiquitinase USP9X deubiquitinates β-catenin and promotes high grade glioma cell growth
Abstract
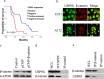
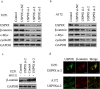
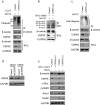
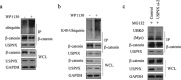
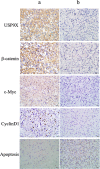
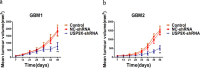
β-catenin is a crucial signal transduction molecule in the Wnt/β-catenin signal pathway, and increased β-catenin expression has consistently been found in high grade gliomas. However, the mechanisms responsible for β-catenin overexpression have remained elusive.Here we show that the deubiquitinase USP9X stabilizes β-catenin and thereby promotes high grade glioma cell growth. USP9X binds β-catenin and removes the Lys 48-linked polyubiquitin chains that normally mark β-catenin for proteasomal degradation. Increased USP9X expression correlates with increased β-catenin protein in high grade glioma tissues. Moreover, patients with high grade glioma overexpressing USP9X have a poor prognosis. Knockdown of USP9X suppresses cell proliferation, inhibits G1/S phase conversion, and induces apoptosis in U251 and A172 cells. Interestingly, c-Myc and cyclinD1, which are important downstream target genes in the Wnt/β-catenin signal pathway, also show decreased expression in cells with siRNA-mediated down-regulation of USP9X. Down-regulation of USP9X also consistently inhibits the tumorigenicity of primary glioma cells in vivo.In summary, these results indicate that USP9X stabilizes β-catenin and activates Wnt/β-catenin signal pathway to promote glioma cell proliferation and survival. USP9X could also potentially be a novel therapeutic target for high grade gliomas.
Keywords: USP9X; deubiquitination; high grade gliomas; prognosis; β-catenin.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

Similar articles
-
Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410.Biosci Rep. 2019 Jan 3;39(1):BSR20180395. doi: 10.1042/BSR20180395. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30498093 Free PMC article.
-
USP9X-mediated deubiquitination of B-cell CLL/lymphoma 9 potentiates Wnt signaling and promotes breast carcinogenesis.J Biol Chem. 2019 Jun 21;294(25):9844-9857. doi: 10.1074/jbc.RA119.007655. Epub 2019 May 9. J Biol Chem. 2019. PMID: 31073027 Free PMC article.
-
Long non-coding RNA H19 regulates the development of gliomas through the Wnt/β-catenin signaling pathway.Eur Rev Med Pharmacol Sci. 2019 May;23(10):4243-4253. doi: 10.26355/eurrev_201905_17929. Eur Rev Med Pharmacol Sci. 2019. PMID: 31173296
-
Wnt/β-catenin signaling cascade: A promising target for glioma therapy.J Cell Physiol. 2019 Mar;234(3):2217-2228. doi: 10.1002/jcp.27186. Epub 2018 Sep 17. J Cell Physiol. 2019. PMID: 30277583 Review.
-
Current advances of long non-coding RNAs mediated by wnt signaling in glioma.Pathol Res Pract. 2020 Aug;216(8):153008. doi: 10.1016/j.prp.2020.153008. Epub 2020 May 25. Pathol Res Pract. 2020. PMID: 32703485 Review.
Cited by
-
Advances in the Development Ubiquitin-Specific Peptidase (USP) Inhibitors.Int J Mol Sci. 2021 Apr 27;22(9):4546. doi: 10.3390/ijms22094546. Int J Mol Sci. 2021. PMID: 33925279 Free PMC article. Review.
-
Usp9X Regulates Cell Death in Malignant Peripheral Nerve Sheath Tumors.Sci Rep. 2018 Nov 26;8(1):17390. doi: 10.1038/s41598-018-35806-5. Sci Rep. 2018. PMID: 30478285 Free PMC article.
-
Protein-Protein Interaction Network Extraction Using Text Mining Methods Adds Insight into Autism Spectrum Disorder.Biology (Basel). 2023 Oct 18;12(10):1344. doi: 10.3390/biology12101344. Biology (Basel). 2023. PMID: 37887054 Free PMC article.
-
Copy number variation of ubiquitin- specific proteases genes in blood leukocytes and colorectal cancer.Cancer Biol Ther. 2020 Jul 2;21(7):637-646. doi: 10.1080/15384047.2020.1750860. Epub 2020 May 4. Cancer Biol Ther. 2020. PMID: 32364424 Free PMC article.
-
Ubiquitin system mutations in neurological diseases.Trends Biochem Sci. 2024 Oct;49(10):875-887. doi: 10.1016/j.tibs.2024.06.011. Epub 2024 Jul 6. Trends Biochem Sci. 2024. PMID: 38972780 Review.
References
-
- Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

