A new humanized in vivo model of KIT D816V+ advanced systemic mastocytosis monitored using a secreted luciferase
- PMID: 27783996
- PMCID: PMC5347747
- DOI: 10.18632/oncotarget.12824
A new humanized in vivo model of KIT D816V+ advanced systemic mastocytosis monitored using a secreted luciferase
Abstract
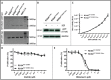
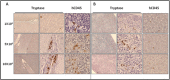
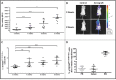
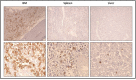
Systemic mastocytosis are rare neoplasms characterized by accumulation of mast cells in at least one internal organ. The majority of systemic mastocytosis patients carry KIT D816V mutation, which activates constitutively the KIT receptor. Patient with advanced forms of systemic mastocytosis, such as aggressive systemic mastocytosis or mast cell leukemia, are poorly treated to date. Unfortunately, the lack of in vivo models reflecting KIT D816V+ advanced disease hampers pathophysiological studies and preclinical development of new therapies for such patients. Here, we describe a new in vivo model of KIT D816V+ advanced systemic mastocytosis developed by transplantation of the human ROSAKIT D816V-Gluc mast cell line in NOD-SCID IL-2R γ-/- mice, using Gaussia princeps luciferase as a reporter. Intravenous injection of ROSAKIT D816V-Gluc cells led, in 4 weeks, to engraftment in all injected primary recipient mice. Engrafted cells were found at high levels in bone marrow, and at lower levels in spleen, liver and peripheral blood. Disease progression was easily monitored by repeated quantification of Gaussia princeps luciferase activity in peripheral blood. This quantification evidenced a linear relationship between the number of cells injected and the neoplastic mast cell burden in mice. Interestingly, the secondary transplantation of ROSAKIT D816V-Gluc cells increased their engraftment capability. To conclude, this new in vivo model mimics at the best the features of human KIT D816V+ advanced systemic mastocytosis. In addition, it is a unique and convenient tool to study the kinetics of the disease and the potential in vivo activity of new drugs targeting neoplastic mast cells.
Keywords: KIT D816V mutant; NSG mice; ROSAKIT D816V cell line; advanced systemic mastocytosis; gluc reporter.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Histone deacetylase inhibitor SAHA mediates mast cell death and epigenetic silencing of constitutively active D816V KIT in systemic mastocytosis.Oncotarget. 2017 Feb 7;8(6):9647-9659. doi: 10.18632/oncotarget.14181. Oncotarget. 2017. PMID: 28038453 Free PMC article.
-
A transgenic zebrafish model expressing KIT-D816V recapitulates features of aggressive systemic mastocytosis.Br J Haematol. 2014 Oct;167(1):48-61. doi: 10.1111/bjh.12999. Epub 2014 Jul 2. Br J Haematol. 2014. PMID: 24989799
-
The KIT and PDGFRA switch-control inhibitor DCC-2618 blocks growth and survival of multiple neoplastic cell types in advanced mastocytosis.Haematologica. 2018 May;103(5):799-809. doi: 10.3324/haematol.2017.179895. Epub 2018 Feb 8. Haematologica. 2018. PMID: 29439183 Free PMC article.
-
Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors.Int J Mol Sci. 2021 Mar 15;22(6):2983. doi: 10.3390/ijms22062983. Int J Mol Sci. 2021. PMID: 33804174 Free PMC article. Review.
-
Clinical Validation of KIT Inhibition in Advanced Systemic Mastocytosis.Curr Hematol Malig Rep. 2018 Oct;13(5):407-416. doi: 10.1007/s11899-018-0469-3. Curr Hematol Malig Rep. 2018. PMID: 30155614 Review.
Cited by
-
Drug delivery targets and strategies to address mast cell diseases.Expert Opin Drug Deliv. 2023 Feb;20(2):205-222. doi: 10.1080/17425247.2023.2166926. Epub 2023 Jan 29. Expert Opin Drug Deliv. 2023. PMID: 36629456 Free PMC article. Review.
-
In vitro and in vivo efficacy of an anti-CD203c conjugated antibody (AGS-16C3F) in mouse models of advanced systemic mastocytosis.Blood Adv. 2019 Feb 26;3(4):633-643. doi: 10.1182/bloodadvances.2018026179. Blood Adv. 2019. PMID: 30804017 Free PMC article.
-
Preclinical human models and emerging therapeutics for advanced systemic mastocytosis.Haematologica. 2018 Nov;103(11):1760-1771. doi: 10.3324/haematol.2018.195867. Epub 2018 Jul 5. Haematologica. 2018. PMID: 29976735 Free PMC article. Review.
-
Mast Cell and Basophil Cell Lines: A Compendium.Methods Mol Biol. 2020;2163:127-144. doi: 10.1007/978-1-0716-0696-4_10. Methods Mol Biol. 2020. PMID: 32766971 Review.
References
-
- Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–5. - PubMed
-
- Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. - PubMed
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. - PubMed
-
- Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107:51–3. - PubMed
-
- Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26:387–405. v. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

