A kidney-selective biopolymer for targeted drug delivery
- PMID: 27784692
- PMCID: PMC5283886
- DOI: 10.1152/ajprenal.00143.2016
A kidney-selective biopolymer for targeted drug delivery
Abstract
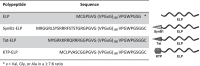
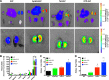
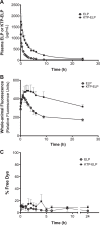
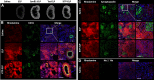
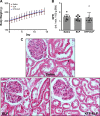
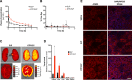
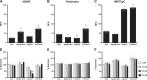
Improving drug delivery to the kidney using renal-targeted therapeutics is a promising but underdeveloped area. We aimed to develop a kidney-targeting construct for renal-specific drug delivery. Elastin-like polypeptides (ELPs) are nonimmunogenic protein-based carriers that can stabilize attached small-molecule and peptide therapeutics. We modified ELP at its NH2-terminus with a cyclic, seven-amino acid kidney-targeting peptide (KTP) and at its COOH-terminus with a cysteine residue for tracer conjugation. Comparative in vivo pharmacokinetics and biodistribution in rat and swine models and in vitro cell binding studies using human renal cells were performed. KTP-ELP had a longer plasma half-life than ELP in both animal models and was similarly accumulated in kidneys at levels fivefold higher than untargeted ELP, showing renal levels 15- to over 150-fold higher than in other major organs. Renal fluorescence histology demonstrated high accumulation of KTP-ELP in proximal tubules and vascular endothelium. Furthermore, a 14-day infusion of a high dose of ELP or KTP-ELP did not affect body weight, glomerular filtration rate, or albuminuria, or induce renal tissue damage compared with saline-treated controls. In vitro experiments showed higher binding of KTP-ELP to human podocytes, proximal tubule epithelial, and glomerular microvascular endothelial cells than untargeted ELP. These results show the high renal selectivity of KTP-ELP, support the notion that the construct is not species specific, and demonstrate that it does not induce acute renal toxicity. The plasticity of ELP for attachment of any class of therapeutics unlocks the possibility of applying ELP technology for targeted treatment of renal disease in future studies.
Keywords: biopolymer; drug delivery; elastin-like polypeptide; kidney targeting peptide; renal disease.
Copyright © 2017 the American Physiological Society.
Figures
Comment in
-
Hitching a ride to renal repair.Am J Physiol Renal Physiol. 2017 Feb 1;312(2):F276-F277. doi: 10.1152/ajprenal.00589.2016. Epub 2016 Nov 9. Am J Physiol Renal Physiol. 2017. PMID: 28153914 No abstract available.
References
-
- American Kidney Fund 2015 Kidney Disease Statistics. Rockville, MD: American Kidney Fund, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

