Glucocorticoid Receptor β Induces Hepatic Steatosis by Augmenting Inflammation and Inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) α
- PMID: 27784782
- PMCID: PMC5203696
- DOI: 10.1074/jbc.M116.752311
Glucocorticoid Receptor β Induces Hepatic Steatosis by Augmenting Inflammation and Inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) α
Abstract
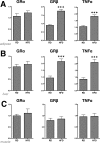
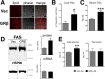
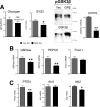
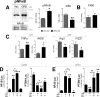
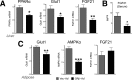
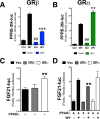
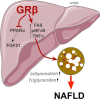
Glucocorticoids (GCs) regulate energy supply in response to stress by increasing hepatic gluconeogenesis during fasting. Long-term GC treatment induces hepatic steatosis and weight gain. GC signaling is coordinated via the GC receptor (GR) GRα, as the GRβ isoform lacks a ligand-binding domain. The roles of the GR isoforms in the regulation of lipid accumulation is unknown. The purpose of this study was to determine whether GRβ inhibits the actions of GCs in the liver, or enhances hepatic lipid accumulation. We show that GRβ expression is increased in adipose and liver tissues in obese high-fat fed mice. Adenovirus-mediated delivery of hepatic GRβ overexpression (GRβ-Ad) resulted in suppression of gluconeogenic genes and hyperglycemia in mice on a regular diet. Furthermore, GRβ-Ad mice had increased hepatic lipid accumulation and serum triglyceride levels possibly due to the activation of NF-κB signaling and increased tumor necrosis factor α (TNFα) and inducible nitric-oxide synthase expression, indicative of enhanced M1 macrophages and the development of steatosis. Consequently, GRβ-Ad mice had increased glycogen synthase kinase 3β (GSK3β) activity and reduced hepatic PPARα and fibroblast growth factor 21 (FGF21) expression and lower serum FGF21 levels, which are two proteins known to increase during fasting to enhance the burning of fat by activating the β-oxidation pathway. In conclusion, GRβ antagonizes the GC-induced signaling during fasting via GRα and the PPARα-FGF21 axis that reduces fat burning. Furthermore, hepatic GRβ increases inflammation, which leads to hepatic lipid accumulation.
Keywords: NAFLD; NASH; PPARα; fatty acid metabolism; fatty acid synthase (FAS); fatty liver; glucocorticoid receptor; hepatic steatosis; inflammation; lipid.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Day C. P., and James O. F. (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114, 842–845 - PubMed
-
- Dunderski J., and Matic G. (2009) Glucocorticoid receptor in health and disease. J. Med. Biochem. 28, 248–261
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

