Short-latency allocentric control of saccadic eye movements
- PMID: 27784804
- PMCID: PMC5236114
- DOI: 10.1152/jn.00451.2016
Short-latency allocentric control of saccadic eye movements
Abstract
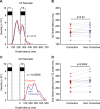
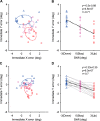
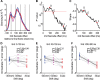
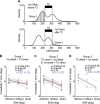
It is generally accepted that the neural circuits that are implicated in saccade control use retinotopically coded target locations. However, several studies have revealed that nonretinotopic representation is also used. This idea raises a question about whether nonretinotopic coding is egocentric (head or body centered) or allocentric (environment centered). In the current study, we hypothesized that allocentric coding may play a crucial role in immediate saccade control. To test this hypothesis, we used an immediate double-step saccade task toward two sequentially flashed targets with a frame in the background, and we examined whether the end point of the second saccade was affected by a transient shift of the background that participants were told to ignore. When the background was shifted transiently upward (or downward) during the flash of the second target, the second saccade generally erred the target downward (or upward), which was in the direction opposite to the shift of the background. The effect on the second saccade became significant within 150 ms after the frame was presented for decoding and was built up for 200 ms thereafter. When the second saccade was not adjusted, a small, corrective saccade followed within 300 ms. The effect scaled linearly with the shift size up to 3° for a noncorrective second saccade and up to 6° for a corrective saccade. The present results show that an allocentric location of a target is rapidly represented by the brain and used for controlling saccades.
New & noteworthy: We found that the saccade end point was shifted from the actual target position toward the direction expected from allocentric coding when a large frame in the background was transiently shifted during the period of target presentation. The effect occurred within 150 ms. The present study provides direct evidence that the brain rapidly uses allocentric coding of a target to control immediate saccades.
Keywords: allocentric coordinate; background frame; saccadic eye movements.
Copyright © 2017 the American Physiological Society.
Figures





References
-
- Aivar MP, Hayhoe MM, Chizk CL, Mruczek RE. Spatial memory and saccadic targeting in a natural task. J Vis 5: 177–193, 2005. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. - PubMed
-
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985. - PubMed
-
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583, 2006. - PubMed
-
- Chafee MV, Averbeck BB, Crowe DA. Representing spatial relationships in posterior parietal cortex: single neurons code object-referenced position. Cereb Cortex 17: 2914–2932, 2007. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

