Osimertinib in the treatment of patients with epidermal growth factor receptor T790M mutation-positive metastatic non-small cell lung cancer: clinical trial evidence and experience
- PMID: 27784815
- PMCID: PMC5933598
- DOI: 10.1177/1753465816670498
Osimertinib in the treatment of patients with epidermal growth factor receptor T790M mutation-positive metastatic non-small cell lung cancer: clinical trial evidence and experience
Abstract
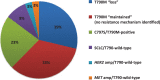
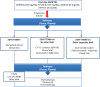
Patients with advanced epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC) are particularly sensitive to treatment with first- or second-generation EGFR tyrosine kinase inhibitors such as gefitinib, erlotinib and afatinib, which block the cell-signaling pathways that drive the growth of tumor cells. Unfortunately, the majority of patients develop resistance to them after a median duration of response of around 10 months, and in over half of these patients the emergence of the EGFR T790M resistance mutation is detected. Osimertinib is an oral, highly selective, irreversible inhibitor of both EGFR-activating mutations and the T790M-resistance mutation, while sparing the activity of wild-type EGFR This article reviews clinical trial development of osimertinib in patients with NSCLC, presenting efficacy and safety evidence for its value in the EGFR T790M mutation-positive population and in different settings, including patients with metastatic disease. The preclinical background of clinically acquired resistance to osimertinib is presented and the combination tactics being investigated in an attempt to circumvent this are addressed.
Keywords: AZD9291; T790M; ctDNA; epidermal growth factor receptor; metastatic; non-small cell lung cancer; osimertinib.
© The Author(s), 2016.
Conflict of interest statement
Figures


References
-
- Ahn M., Tsai C., Yang J., Shepherd F., Satouchi M., Kim D., et al. (2015) AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from phase II studies. Eur J Cancer 51: S625–S626.
-
- Ahn M., Yang J., Yu H., Saka H., Ramalingam H., Huang X. (2016) Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. Sixth IASLC/ESMO. Eur Lung Cancer Conf 11: S115; abstract LBA2.
-
- Barlesi F., Mazieres J., Merlio J., Debieuvre D., Mosser J., Lena H., et al. (2016) Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet Oncol 387: 1415–1426. - PubMed
-
- Chamberlain M., Kormanik P. (1998) Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol 5: 506–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

