Preventive effect of zoledronic acid on aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole
- PMID: 27785049
- PMCID: PMC5063560
- DOI: 10.2147/OTT.S115058
Preventive effect of zoledronic acid on aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole
Abstract
Background: This study aims to compare the efficacy and safety between zoledronic acid combined with calcium and calcium alone to prevent aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole.
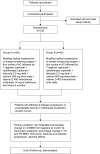
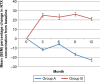
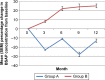
Methods: One hundred twenty patients were randomly divided into two groups, A and B. Patients in group A (n=60) received modified radical mastectomy or breast-conserving surgery + four cycles of AC followed by T regimen (optional) + radiotherapy (optional) + letrozole 2.5 mg daily + calcium 500 mg twice daily + vitamin D 400 international units daily +4 mg of zoledronic acid every 6 months, while patients in group B (n=60) were not given zoledronic acid and the rest of the treatments of group B were the same as group A. All the patients were followed up for 1 year. The primary endpoint was the intrapatient percentage change in lumbar spine (LS) bone mineral density (BMD) from baseline to month 12. Secondary endpoints included the percentage change in total hip (TH) and femoral neck (FN) BMD, the incidence of osteoporosis, the incidence of a clinically meaningful 5% decline in BMD at 1 year, change of serum N-telopeptide of type 1 collagen (NTX) and bone-specific alkaline phosphatase (BSAP) concentrations.
Results: Patients in group A had a statistically significant higher average change and average percent change in LS, FN, and TH than group B. Group A had a statistically significant lower incidence of a clinically meaningful loss of bone density at the LS, FN, or TH than Group B. The incidence of osteoporosis in group A was significantly lower than group B. The decreases in NTX and BSAP concentrations from baseline to month 12 in patients of group A were significant; in contrast, patients in group B were found to have increases in NTX and BSAP concentrations from baseline. The most common adverse reactions in patients are flu-like symptoms (38%), bone pain (28%), and joint pain (20%).
Conclusion: AI-associated bone loss can be prevented by concurrent zoledronic acid for postmenopausal breast cancer patients.
Keywords: breast cancer; letrozole; postmenopausal osteoporosis; zoledronic acid.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2013;62(1):10–29. - PubMed
-
- Regan MM, Price KN. Giobbie-Hurder A, Thürlimann B, Gelber RD; Interpreting Breast international group (BIG) 1-98: a randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer. Breast Cancer Res. 2011;13(3):209. - PMC - PubMed
-
- Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366(9484):455–462. - PubMed
-
- Llombart A, Frassoldati A, Paija O, et al. Immediate administration of Zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12(1):40–48. - PubMed
-
- Brufsky A, Harker G, Beck J, et al. The effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the Z-FAST Study 5-Year final follow-Up. Cancer Res. 2010;69(24 Suppl):4083–4083.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

