Systematic review: treatment pattern and clinical effectiveness and safety of pharmaceutical therapies for Crohn's disease in Europe
- PMID: 27785086
- PMCID: PMC5063598
- DOI: 10.2147/CEG.S109696
Systematic review: treatment pattern and clinical effectiveness and safety of pharmaceutical therapies for Crohn's disease in Europe
Abstract
Background: Although many clinical trials have been conducted in treatments of Crohn's disease (CD), whether the trial results were representative of daily practice needs to be supported by studies conducted in real-world settings.
Aim: This study aims to identify how CD is treated and what are the clinical effectiveness and safety of the pharmaceutical therapies of CD in real-world settings.
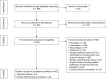
Methods: A systematic literature review was conducted based on Medline®, Embase®, and Cochrane. All publications were assessed for title/abstract and full-text according to a predefined study protocol. Data were extracted and reported.
Results: A total of 1,998 publications were identified. Fifty studies including six publications reporting treatment pattern and 44 studies reporting clinical effectiveness and safety of pharmaceutical therapies in CD management in Europe were included. 5-Aminosalicylic acid and corticosteroids were reported to be used among 14%-74% of CD patients. Immunomodulators were used by 14%-25% and 29%-31% of CD patients as an initial and follow-up treatment, respectively. Biological therapies were used by 25%-33% of CD patients. A trend toward an increasing use of immunomodulators and biological therapies in Europe has been reported in recent years. Approximately 50% of patients achieved remission on immunomodulator or biologic treatment, although a relapse rate of up to 23% has been reported.
Conclusion: There is a trend of treatment shift to immunomodulators and biologics in CD management. Clinical effectiveness of immunomodulators and biologics has been demonstrated, though with a lack of sustainability of the effectiveness.
Keywords: biologics; dose-escalation; immunomodulators; inflammatory bowel disease; real world evidence.
Conflict of interest statement
Weiwei Xu is employed by Pharmerit International. Filippo Lelli, Solomon Nuhoho, and Xin Ying Lee are employed by Janssen Pharmaceutical affiliates. The authors report no conflicts of interest in this work.
Figures
References
-
- Mayberry JF, Lobo A, Ford AC, Thomas A. NICE clinical guideline (CG152): the management of Crohn’s disease in adults, children and young people. Aliment Pharmacol Ther. 2013;37(2):195–203. - PubMed
-
- Carpio D, Barreiro-de Acosta M, Echarri A, et al. Influence of urban/rural and coastal/inland environment on the prevalence, phenotype, and clinical course of inflammatory bowel disease patients from northwest of Spain: a cross-sectional study. Eur J Gastroenterol Hepatol. 2015;27(9):1030–1037. - PubMed
-
- Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohn’s Colitis. 2010;4(1):28–62. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources