Reflectance confocal microscopy of skin in vivo: From bench to bedside
- PMID: 27785781
- PMCID: PMC5575825
- DOI: 10.1002/lsm.22600
Reflectance confocal microscopy of skin in vivo: From bench to bedside
Abstract
Following more than two decades of effort, reflectance confocal microscopy (RCM) imaging of skin was granted codes for reimbursement by the US Centers for Medicare and Medicaid Services. Dermatologists in the USA have started billing and receiving reimbursement for the imaging procedure and for the reading and interpretation of images. RCM imaging combined with dermoscopic examination is guiding the triage of lesions into those that appear benign, which are being spared from biopsy, against those that appear suspicious, which are then biopsied. Thus far, a few thousand patients have been spared from biopsy of benign lesions. The journey of RCM imaging from bench to bedside is certainly a success story, but still much more work lies ahead toward wider dissemination, acceptance, and adoption. We present a brief review of RCM imaging and highlight key challenges and opportunities. The success of RCM imaging paves the way for other emerging optical technologies, as well-and our bet for the future is on multimodal approaches. Lasers Surg. Med. 49:7-19, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: melanoma; non-melanoma; reflectance confocal microscopy; reimbursement codes; skin; skin cancer.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Milind Rajadhyaksha is a former employee of, and owns equity in, Caliber Imaging and Diagnostics (formerly, Lucid Inc.), the company that manufactures and sells the VivaScope reflectance confocal microscope. The VivaScope is the commercial version of an original laboratory prototype that was developed by Rajadhyaksha when he was at Wellman Laboratories of Photomedicine, Massachusetts General Hospital, Harvard Medical School. Allan Halpern serves on the scientific advisory board of Caliber Imaging and Diagnostics. None for Ashfaq Marghoob, Anthony Rossi and Kishwer Nehal.
Figures
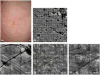
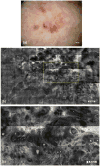

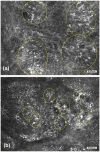
References
-
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianF....
-
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States 2006. Arch Dermatol. 2010;146:283–287. - PubMed
-
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. - PubMed
-
- Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. - PubMed
-
- Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin. 2010;60:301–316. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

