Exploration of Biomarkers for Amoxicillin/Clavulanate-Induced Liver Injury: Multi-Omics Approaches
- PMID: 27785887
- PMCID: PMC5421739
- DOI: 10.1111/cts.12425
Exploration of Biomarkers for Amoxicillin/Clavulanate-Induced Liver Injury: Multi-Omics Approaches
Abstract
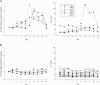
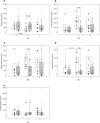
To explore potential biomarkers for amoxicillin/clavulanate-induced liver injury (AC-DILI), we conducted a clinical trial in 32 healthy subjects based on multi-omics approaches. Every subject was administered amoxicillin/clavulanate for 14 days. The liver-specific microRNA-122 (miR-122) level increased prior to and correlated well with the observed alanine aminotransferase (ALT) level increase. This result indicates its potential as a sensitive early marker for AC-DILI. We also identified urinary metabolites, such as azelaic acid and 7-methylxanthine, with levels that significantly differed among the groups classified by ALT elevation level on day 8 after drug administration (P < 0.05). Lymphocyte proliferation in response to the drug was also observed. These findings demonstrate sequential changes in the process of AC-DILI, including metabolic changes, increased miR-122 level, increased liver enzyme activity, and enhanced lymphocyte proliferation after drug administration. In conclusion, this study provides potential biomarkers for AC-DILI based on currently known mechanisms using comprehensive multi-omics approaches.
© 2016 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures



Similar articles
-
Drug-induced liver injury.Mayo Clin Proc. 2014 Jan;89(1):95-106. doi: 10.1016/j.mayocp.2013.09.016. Mayo Clin Proc. 2014. PMID: 24388027 Review.
-
Amoxicillin-Clavulanate-Induced Liver Injury.Dig Dis Sci. 2016 Aug;61(8):2406-2416. doi: 10.1007/s10620-016-4121-6. Epub 2016 Mar 22. Dig Dis Sci. 2016. PMID: 27003146 Free PMC article.
-
Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate-induced liver injury.Hepatology. 2015 Sep;62(3):887-99. doi: 10.1002/hep.27912. Epub 2015 Jul 23. Hepatology. 2015. PMID: 25998949
-
Predicting and preventing acute drug-induced liver injury: what's new in 2010?Expert Opin Drug Metab Toxicol. 2010 Sep;6(9):1047-61. doi: 10.1517/17425255.2010.503706. Expert Opin Drug Metab Toxicol. 2010. PMID: 20615079 Review.
-
Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts.Liver Int. 2014 Mar;34(3):367-78. doi: 10.1111/liv.12322. Epub 2013 Oct 14. Liver Int. 2014. PMID: 24118944
Cited by
-
An insight into pharmacokinetics and dose optimization of antimicrobials agents in elderly patients.Front Pharmacol. 2024 Sep 30;15:1396994. doi: 10.3389/fphar.2024.1396994. eCollection 2024. Front Pharmacol. 2024. PMID: 39403139 Free PMC article. Review.
-
Human quad liver-on-chip system as a tool toward bridging the gap between animals and humans regarding toxicology and pharmacology of a cannabidiol-rich cannabis extract.Drug Chem Toxicol. 2025 May;48(3):578-585. doi: 10.1080/01480545.2024.2388292. Epub 2024 Aug 19. Drug Chem Toxicol. 2025. PMID: 39155655
-
Drug-Induced Liver Injury: Highlights of the Recent Literature.Drug Saf. 2019 Mar;42(3):365-387. doi: 10.1007/s40264-018-0743-2. Drug Saf. 2019. PMID: 30343418 Review.
-
Role of Ursodeoxycholic Acid in Treating and Preventing Idiosyncratic Drug-Induced Liver Injury. A Systematic Review.Front Pharmacol. 2021 Oct 27;12:744488. doi: 10.3389/fphar.2021.744488. eCollection 2021. Front Pharmacol. 2021. PMID: 34776963 Free PMC article. Review.
-
The Potential Role of Metabolomics in Drug-Induced Liver Injury (DILI) Assessment.Metabolites. 2022 Jun 19;12(6):564. doi: 10.3390/metabo12060564. Metabolites. 2022. PMID: 35736496 Free PMC article. Review.
References
-
- Qureshi, Z.P. , Seoane‐Vazquez, E. , Rodriguez‐Monguio, R. , Stevenson, K.B. & Szeinbach, S.L. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 20, 772–777 (2011). - PubMed
-
- Regev, A. & Bjornsson, E.S. Drug‐induced liver injury: morbidity, mortality, and Hy's law. Gastroenterology 147, 20–24 (2014). - PubMed
-
- Kong, A.P. et al Independent associations of alanine aminotransferase (ALT) levels with cardiovascular risk factor clustering in Chinese adolescents. J. Hepatol. 49, 115–122 (2008). - PubMed
-
- Lee, J.K. et al Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology 51, 1577–1583 (2010). - PubMed
-
- Dufour, D.R. Alanine aminotransferase: is it healthy to be “normal”? Hepatology 50, 1699–1701 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

