Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction
- PMID: 27786170
- PMCID: PMC5095349
- DOI: 10.1038/ncomms13306
Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction
Abstract
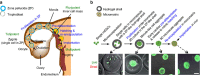
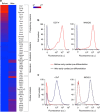
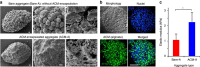
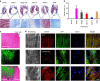
It is difficult to achieve minimally invasive injectable cell delivery while maintaining high cell retention and animal survival for in vivo stem cell therapy of myocardial infarction. Here we show that pluripotent stem cell aggregates pre-differentiated into the early cardiac lineage and encapsulated in a biocompatible and biodegradable micromatrix, are suitable for injectable delivery. This method significantly improves the survival of the injected cells by more than six-fold compared with the conventional practice of injecting single cells, and effectively prevents teratoma formation. Moreover, this method significantly enhances cardiac function and survival of animals after myocardial infarction, as a result of a localized immunosuppression effect of the micromatrix and the in situ cardiac regeneration by the injected cells.
Conflict of interest statement
X.H. disclosed the idea reported in this work to the Technology and Commercialization Office at The Ohio State University. N.W. is a Co-Founder and Chief Scientific Officer at TRIM-edicine, Inc. and has no competing financial interests on this study. The remaining authors declare no competing financial interests.
Figures

References
-
- Mozaffarian D. et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360 (2016). - PubMed
-
- Laslett L. J. et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J. Am. Coll. Cardiol. 60, S1–49 (2012). - PubMed
-
- Segers V. F. & Lee R. T. Stem-cell therapy for cardiac disease. Nature 451, 937–942 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

