Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release
- PMID: 27786259
- PMCID: PMC5081536
- DOI: 10.1038/srep36016
Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release
Abstract
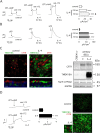
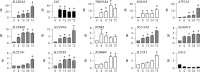
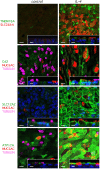
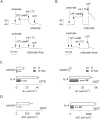
Goblet cell hyperplasia, a feature of asthma and other respiratory diseases, is driven by the Th-2 cytokines IL-4 and IL-13. In human bronchial epithelial cells, we find that IL-4 induces the expression of many genes coding for ion channels and transporters, including TMEM16A, SLC26A4, SLC12A2, and ATP12A. At the functional level, we find that IL-4 enhances calcium- and cAMP-activated chloride/bicarbonate secretion, resulting in high bicarbonate concentration and alkaline pH in the fluid covering the apical surface of epithelia. Importantly, mucin release, elicited by purinergic stimulation, requires the presence of bicarbonate in the basolateral solution and is defective in cells derived from cystic fibrosis patients. In conclusion, our results suggest that Th-2 cytokines induce a profound change in expression and function in multiple ion channels and transporters that results in enhanced bicarbonate transport ability. This change is required as an important mechanism to favor release and clearance of mucus.
Figures




Similar articles
-
Relationship between calcium-activated chloride channel 1 and MUC5AC in goblet cell hyperplasia induced by interleukin-13 in human bronchial epithelial cells.Respiration. 2006;73(3):347-59. doi: 10.1159/000091391. Epub 2006 Feb 6. Respiration. 2006. PMID: 16465045
-
Increased expression of ATP12A proton pump in cystic fibrosis airways.JCI Insight. 2018 Oct 18;3(20):e123616. doi: 10.1172/jci.insight.123616. JCI Insight. 2018. PMID: 30333310 Free PMC article.
-
Model systems for investigating mucin gene expression in airway diseases.J Aerosol Med. 2000 Fall;13(3):245-61. doi: 10.1089/jam.2000.13.245. J Aerosol Med. 2000. PMID: 11066028
-
Transepithelial ion transport across duct cells of the salivary gland.Oral Dis. 2015 Oct;21(7):826-35. doi: 10.1111/odi.12201. Epub 2013 Nov 25. Oral Dis. 2015. PMID: 24164806 Review.
-
Airway goblet cells: responsive and adaptable front-line defenders.Eur Respir J. 1994 Sep;7(9):1690-706. Eur Respir J. 1994. PMID: 7995400 Review.
Cited by
-
The Non-Gastric H+/K+ ATPase (ATP12A) Is Expressed in Mammalian Spermatozoa.Int J Mol Sci. 2022 Jan 19;23(3):1048. doi: 10.3390/ijms23031048. Int J Mol Sci. 2022. PMID: 35162971 Free PMC article.
-
A causal effects of neutrophil extracellular traps and its biomarkers on acute respiratory distress syndrome: a two-sample Mendelian randomization study.Sci Rep. 2025 Apr 8;15(1):11995. doi: 10.1038/s41598-025-95676-6. Sci Rep. 2025. PMID: 40199908 Free PMC article.
-
Airway surface hyperviscosity and defective mucociliary transport by IL-17/TNF-α are corrected by β-adrenergic stimulus.JCI Insight. 2022 Nov 22;7(22):e164944. doi: 10.1172/jci.insight.164944. JCI Insight. 2022. PMID: 36219481 Free PMC article.
-
Putting bicarbonate on the spot: pharmacological insights for CFTR correction in the airway epithelium.Front Pharmacol. 2023 Dec 11;14:1293578. doi: 10.3389/fphar.2023.1293578. eCollection 2023. Front Pharmacol. 2023. PMID: 38149052 Free PMC article.
-
Recent advances in developing therapeutics for cystic fibrosis.Hum Mol Genet. 2018 Aug 1;27(R2):R173-R186. doi: 10.1093/hmg/ddy188. Hum Mol Genet. 2018. PMID: 30060192 Free PMC article. Review.
References
-
- Riordan J. R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 (2008). - PubMed
-
- Matsui H. et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95, 1005–1015 (1998). - PubMed
-
- Boucher R. C. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

