Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo
- PMID: 27787519
- PMCID: PMC5133996
- DOI: 10.1038/cddis.2016.334
Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo
Abstract
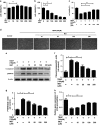
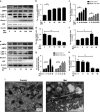
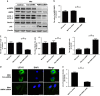
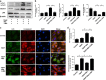
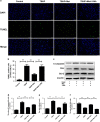
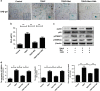
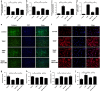
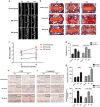
Intervertebral disc degeneration (IDD) is a complicated process that involves both cellular apoptosis and senescence. Metformin has been reported to stimulate autophagy, whereas autophagy is shown to protect against apoptosis and senescence. Therefore, we hypothesize that metformin may have therapeutic effect on IDD through autophagy stimulation. The effect of metformin on IDD was investigated both in vitro and in vivo. Our study showed that metformin attenuated cellular apoptosis and senescence induced by tert-butyl hydroperoxide in nucleus pulposus cells. Autophagy, as well as its upstream regulator AMPK, was activated by metformin in nucleus pulposus cells in a dose- and time-dependent manner. Inhibition of autophagy by 3-MA partially abolished the protective effect of metformin against nucleus pulposus cells' apoptosis and senescence, indicating that autophagy was involved in the protective effect of metformin on IDD. In addition, metformin was shown to promote the expression of anabolic genes such as Col2a1 and Acan expression while inhibiting the expression of catabolic genes such as Mmp3 and Adamts5 in nucleus pulposus cells. In vivo study illustrated that metformin treatment could ameliorate IDD in a puncture-induced rat model. Thus, our study showed that metformin could protect nucleus pulposus cells against apoptosis and senescence via autophagy stimulation and ameliorate disc degeneration in vivo, revealing its potential to be a therapeutic agent for IDD.
Figures
Similar articles
-
Restoration of Autophagic Flux Rescues Oxidative Damage and Mitochondrial Dysfunction to Protect against Intervertebral Disc Degeneration.Oxid Med Cell Longev. 2019 Dec 30;2019:7810320. doi: 10.1155/2019/7810320. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31976028 Free PMC article.
-
Spermidine promotes nucleus pulposus autophagy as a protective mechanism against apoptosis and ameliorates disc degeneration.J Cell Mol Med. 2018 Jun;22(6):3086-3096. doi: 10.1111/jcmm.13586. Epub 2018 Mar 25. J Cell Mol Med. 2018. PMID: 29575654 Free PMC article.
-
β‑ecdysterone protects against apoptosis by promoting autophagy in nucleus pulposus cells and ameliorates disc degeneration.Mol Med Rep. 2019 Mar;19(3):2440-2448. doi: 10.3892/mmr.2019.9861. Epub 2019 Jan 15. Mol Med Rep. 2019. PMID: 30664184
-
Metformin prevents the onset and progression of intervertebral disc degeneration: New insights and potential mechanisms (Review).Int J Mol Med. 2024 Aug;54(2):71. doi: 10.3892/ijmm.2024.5395. Epub 2024 Jul 4. Int J Mol Med. 2024. PMID: 38963023 Free PMC article. Review.
-
M6A methylation-regulated autophagy may be a new therapeutic target for intervertebral disc degeneration.Cell Biol Int. 2024 Apr;48(4):389-403. doi: 10.1002/cbin.12135. Epub 2024 Feb 5. Cell Biol Int. 2024. PMID: 38317355 Review.
Cited by
-
POSTN knockdown suppresses IL-1β-induced inflammation and apoptosis of nucleus pulposus cells via inhibiting the NF-κB pathway and alleviates intervertebral disc degeneration.J Cell Commun Signal. 2024 May 7;18(2):e12030. doi: 10.1002/ccs3.12030. eCollection 2024 Jun. J Cell Commun Signal. 2024. PMID: 38946726 Free PMC article.
-
Selenium Attenuates TBHP-Induced Apoptosis of Nucleus Pulposus Cells by Suppressing Mitochondrial Fission through Activating Nuclear Factor Erythroid 2-Related Factor 2.Oxid Med Cell Longev. 2022 Apr 11;2022:7531788. doi: 10.1155/2022/7531788. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450408 Free PMC article.
-
Cellular senescence and disrupted proteostasis induced by myotube atrophy are prevented with low-dose metformin and leucine cocktail.Aging (Albany NY). 2023 Mar 20;15(6):1808-1832. doi: 10.18632/aging.204600. Epub 2023 Mar 20. Aging (Albany NY). 2023. PMID: 36947713 Free PMC article.
-
Investigation into the anti-inflammatory properties of metformin in intervertebral disc cells.JOR Spine. 2022 Mar 10;5(2):e1197. doi: 10.1002/jsp2.1197. eCollection 2022 Jun. JOR Spine. 2022. PMID: 35783910 Free PMC article.
-
Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model.Int J Biol Sci. 2018 Apr 30;14(6):682-692. doi: 10.7150/ijbs.24081. eCollection 2018. Int J Biol Sci. 2018. PMID: 29904282 Free PMC article.
References
-
- Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine 2000; 25: 487–492. - PubMed
-
- Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am 1991; 22: 263–271. - PubMed
-
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine 2006; 31: 2151–2161. - PubMed
-
- Ding F, Shao Z-w, Xiong L-m. Cell death in intervertebral disc degeneration. Apoptosis 2013; 18: 777–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

