Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial
- PMID: 27787547
- PMCID: PMC5824229
- DOI: 10.1001/jamaoncol.2016.4419
Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial
Abstract
Importance: Girentuximab is a chimeric monoclonal antibody that binds carbonic anhydrase IX, a cell surface glycoprotein ubiquitously expressed in clear cell renal cell carcinoma (ccRCC). Its safety and activity in phase 2 studies prompted investigation into its use as adjuvant monotherapy in participants with high-risk ccRCC.
Objective: To evaluate the safety and efficacy of adjuvant girentuximab on disease-free survival (DFS) and overall survival (OS) in patients with localized completely resected high-risk ccRCC.
Design, setting, and participants: The ARISER trial (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Nonmetastatic RCC) was a randomized, double-blind, placebo-controlled phase 3 clinical trial that took place between June 10, 2004, and April 2, 2013, at 142 academic medical centers in 15 countries in North and South America and Europe. Eligible adult patients had undergone partial or radical nephrectomy for histologically confirmed ccRCC and fell into 1 of the following high-risk groups: pT3/pT4Nx/N0M0 or pTanyN+M0 or pT1b/pT2Nx/N0M0 with nuclear grade 3 or greater. Patients were assigned via central computerized double-blind 1:1 randomization to receive either a single loading dose of girentuximab, 50 mg (week 1), followed by weekly intravenous infusions of girentuximab, 20 mg (weeks 2-24), or placebo, stratified by risk group and region. The data were analyzed from March 31, 2012, to April 2, 2013.
Main outcomes and measures: Co-primary end points were DFS and OS, based on imaging studies assessed by independent radiological review committee. Secondary end points included safety, assessed as the rate and grade of adverse events.
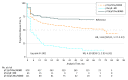
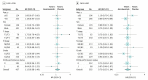
Results: A total of 864 patients (66% male; median [interquartile range] age, 58 [51-65] years) were randomized to girentuximab (n = 433) or placebo (n = 431). Compared with placebo, participants treated with girentuximab had no statistically significant DFS (hazard ratio, 0.97; 95% CI, 0.79-1.18) or OS advantage (hazard ratio, 0.99; 95% CI, 0.74-1.32). Median DFS was 71.4 months (interquartile range, 3 months to not reached) for girentuximab and never reached for placebo group. Median OS was never reached regardless of treatment. Drug-related adverse events occurred in 185 patients (21.6%), reported comparably between arms. Serious adverse events occurred in 72 patients (8.4%), reported comparably between arms. One drug-related serious adverse event occurred in a patient receiving placebo.
Conclusions and relevance: Girentuximab had no clinical benefit as adjuvant treatment for patients with high-risk ccRCC. The surprisingly long DFS and OS in these patients represent a challenge to adjuvant ccRCC drug development.
Trial registration: clinicaltrials.gov Identifier: NCT00087022.
Conflict of interest statement
Figures

Comment in
-
Perioperative Management of Localized Kidney Cancer: Watching and Waiting.JAMA Oncol. 2017 Jul 1;3(7):895-896. doi: 10.1001/jamaoncol.2016.4409. JAMA Oncol. 2017. PMID: 27787546 No abstract available.
-
Girentuximab bringt keinen klinischen Benefit.Aktuelle Urol. 2017 Sep;48(5):419. doi: 10.1055/s-0043-118396. Epub 2017 Aug 30. Aktuelle Urol. 2017. PMID: 28854471 German. No abstract available.
References
-
- Sorbellini M, Kattan MW, Snyder ME, et al. . A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173(1):48-51. - PubMed
-
- Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Fyfe G. High-dose aldesleukin in renal cell carcinoma: long-term survival update. Cancer J Sci Am. 1997;3(suppl 1):S70-S72. - PubMed
-
- Jocham D, Richter A, Hoffmann L, et al. . Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363(9409):594-599. - PubMed
-
- Fishman M, Antonia S. Specific antitumour vaccine for renal cancer. Lancet. 2004;363(9409):583-584. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

