Trial of Amitriptyline, Topiramate, and Placebo for Pediatric Migraine
- PMID: 27788026
- PMCID: PMC5226887
- DOI: 10.1056/NEJMoa1610384
Trial of Amitriptyline, Topiramate, and Placebo for Pediatric Migraine
Abstract
Background: Which medication, if any, to use to prevent the headache of pediatric migraine has not been established.
Methods: We conducted a randomized, double-blind, placebo-controlled trial of amitriptyline (1 mg per kilogram of body weight per day), topiramate (2 mg per kilogram per day), and placebo in children and adolescents 8 to 17 years of age with migraine. Patients were randomly assigned in a 2:2:1 ratio to receive one of the medications or placebo. The primary outcome was a relative reduction of 50% or more in the number of headache days in the comparison of the 28-day baseline period with the last 28 days of a 24-week trial. Secondary outcomes were headache-related disability, headache days, number of trial completers, and serious adverse events that emerged during treatment.
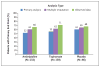
Results: A total of 361 patients underwent randomization, and 328 were included in the primary efficacy analysis (132 in the amitriptyline group, 130 in the topiramate group, and 66 in the placebo group). The trial was concluded early for futility after a planned interim analysis. There were no significant between-group differences in the primary outcome, which occurred in 52% of the patients in the amitriptyline group, 55% of those in the topiramate group, and 61% of those in the placebo group (amitriptyline vs. placebo, P=0.26; topiramate vs. placebo, P=0.48; amitriptyline vs. topiramate, P=0.49). There were also no significant between-group differences in headache-related disability, headache days, or the percentage of patients who completed the 24-week treatment period. Patients who received amitriptyline or topiramate had higher rates of several adverse events than those receiving placebo, including fatigue (30% vs. 14%) and dry mouth (25% vs. 12%) in the amitriptyline group and paresthesia (31% vs. 8%) and weight loss (8% vs. 0%) in the topiramate group. Three patients in the amitriptyline group had serious adverse events of altered mood, and one patient in the topiramate group had a suicide attempt.
Conclusions: There were no significant differences in reduction in headache frequency or headache-related disability in childhood and adolescent migraine with amitriptyline, topiramate, or placebo over a period of 24 weeks. The active drugs were associated with higher rates of adverse events. (Funded by the National Institutes of Health; CHAMP ClinicalTrials.gov number, NCT01581281 ).
Figures
Comment in
-
Pediatric Migraine Headache - Still Searching for Effective Treatments.N Engl J Med. 2017 Jan 12;376(2):169-170. doi: 10.1056/NEJMe1614628. N Engl J Med. 2017. PMID: 28076719 No abstract available.
-
Amitriptyline and topiramate are no better than placebo for childhood migraine.Arch Dis Child Educ Pract Ed. 2017 Dec;102(6):332. doi: 10.1136/archdischild-2017-312734. Epub 2017 Feb 28. Arch Dis Child Educ Pract Ed. 2017. PMID: 28246124 No abstract available.
-
Treatment of Pediatric Migraine.N Engl J Med. 2017 Apr 6;376(14):1388. doi: 10.1056/NEJMc1701674. N Engl J Med. 2017. PMID: 28379805 No abstract available.
-
Treatment of Pediatric Migraine.N Engl J Med. 2017 Apr 6;376(14):1387-8. doi: 10.1056/NEJMc1701674. N Engl J Med. 2017. PMID: 28382806 Free PMC article. No abstract available.
-
Amitriptyline and topiramate do not demonstrate benefit in pediatric migraine.J Pediatr. 2017 Jul;186:209-212. doi: 10.1016/j.jpeds.2017.04.020. J Pediatr. 2017. PMID: 28648276 No abstract available.
-
Screen time associated with adolescent obesity and obesity risk factors.J Pediatr. 2017 Jul;186:209-212. doi: 10.1016/j.jpeds.2017.04.023. J Pediatr. 2017. PMID: 28648277 No abstract available.
-
PURL: Treating migraines: It's different for kids.J Fam Pract. 2018 Apr;67(4):238;239;241. J Fam Pract. 2018. PMID: 29614145 Free PMC article.
References
-
- Stewart WF, Linet MS, Celentano DD, Van Natta M, Ziegler D. Age- and sex-specific incidence rates of migraine with and without visual aura. Am J Epidemiol. 1991;134:1111–20. - PubMed
-
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–9. - PubMed
-
- Stewart WF, Ricci JA, Chee E, Morgan-stein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54. - PubMed
-
- Bille B. A 40-year follow-up of school children with migraine. Cephalalgia. 1997;17:488–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
