In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation
- PMID: 27788249
- PMCID: PMC5082868
- DOI: 10.1371/journal.pone.0165575
In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation
Abstract
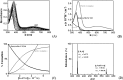
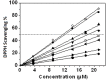
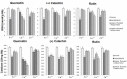
Natural flavonoids such as quercetin, (+)catechin and rutin as well as four methoxylated derivatives of quercetin used as models were investigated to elucidate their impact on the oxidant and antioxidant status of human red blood cells (RBCs). The impact of these compounds against metal toxicity was studied as well as their antiradical activities with DPPH assay. Antihemolytic experiments were conducted on quercetin, (+)catechin and rutin with excess of Fe, Cu and Zn (400 μM), and the oxidant (malondialdehyde, carbonyl proteins) and antioxidant (reduced glutathione, catalase activity) markers were evaluated. The results showed that Fe and Zn have the highest prooxidant effect (37 and 33% of hemolysis, respectively). Quercetin, rutin and (+)catechin exhibited strong antioxidant properties toward Fe, but this effect was decreased with respect to Zn ions. However, the Cu showed a weak antioxidant effect at the highest flavonoid concentration (200 μM), while a prooxidant effect was observed at the lowest flavonoid concentration (100 μM). These results are in agreement with the physico-chemical and antiradical data which demonstrated that binding of the metal ions (for FeNTA: (+)Catechin, KLFeNTA = 1.6(1) × 106 M-1 > Rutin, KLFeNTA = 2.0(9) × 105 M-1 > Quercetin, KLFeNTA = 1.0(7) × 105 M-1 > Q35OH, KLFeNTA = 6.3(8.7) × 104 M-1 > Quercetin3'4'OH and Quercetin 3OH, KLFeNTA ~ 2 × 104 M-1) reflects the (anti)oxidant status of the RBCs. This study reveals that flavonoids have both prooxidant and antioxidant activity depending on the nature and concentration of the flavonoids and metal ions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Correa J, Souza I, Ladeira A, Young M, Aragushi M. Allelopathic potential of Eupatorium maximiliani Schrad. leaves. Allelopathy J. 2000;7:225–34.
-
- Lourenço RMDC, da Silva Melo P, de Almeida ABA. Flavonoids as antifungal agents Antifungal Metabolites from Plants: Springer; 2013. p. 283–300.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

